Abstract
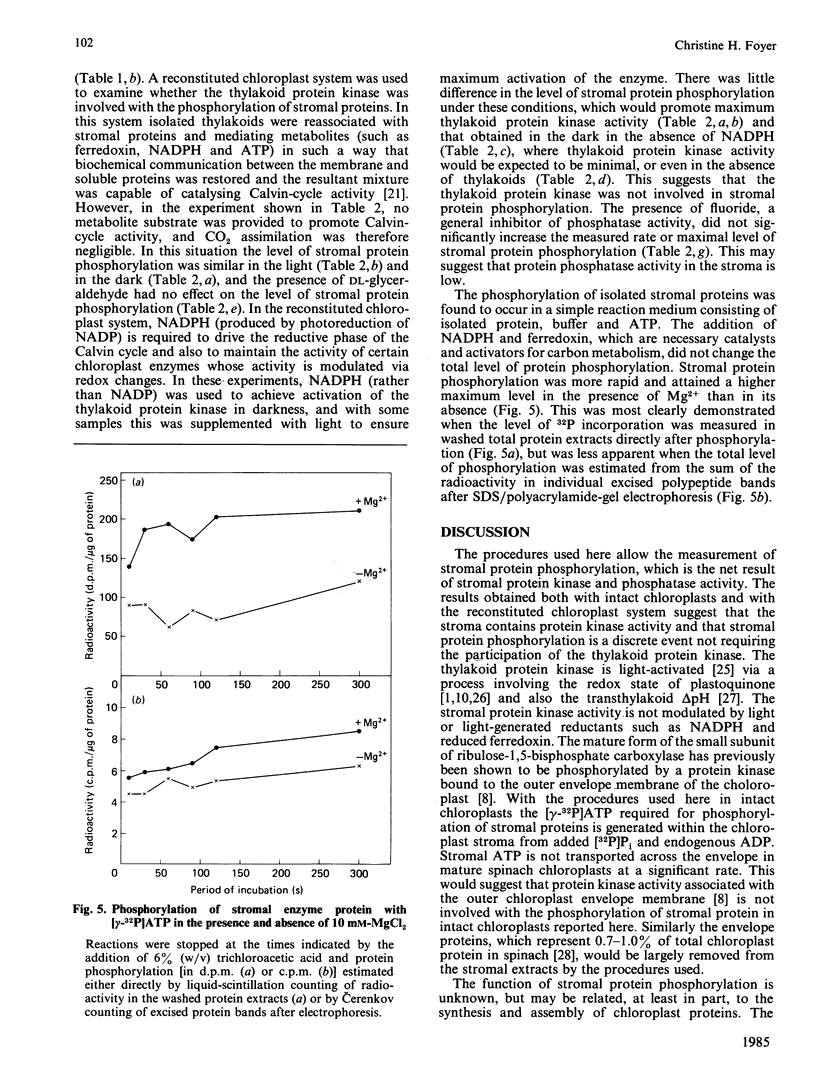
When intact spinach chloroplasts were supplied with [32P]Pi, stromal protein phosphorylation was found to occur in the dark. On illumination the thylakoid protein kinase was activated and the amount of label found in thylakoid proteins quickly exceeded that incorporated into stromal protein, such that the latter was found to account for only 10-15% of the total radioactivity bound to chloroplast proteins after 5 min illumination. The rate of phosphorylation of stromal polypeptides was unchanged by light. After SDS/polyacrylamide-gel electrophoresis, more than 15 labelled polypeptides of stromal origin were observed. A polypeptide with an Mr of approx. 70 000 had the highest specific activity of labelling. Both the large and small subunits of the ribulose-1,5-bisphosphate carboxylase were phosphorylated. The level of phosphorylation of stromal protein was increased by CO2 fixation in intact chloroplasts. This increase was not observed in the absence of NaHCO3 or in the presence of the phosphoribulokinase inhibitor DL-glyceraldehyde. These effects appeared to be largely due to changes in the phosphorylation state of the large and small subunits of ribulose-1,5-bisphosphate carboxylase. Studies with the reconstituted chloroplast system showed that the thylakoid protein kinase(s) played no part in the phosphorylation of stromal protein. The rate and level of phosphorylation of stromal protein was unaffected by the activation state of the thylakoid protein kinase and was unchanged when thylakoids were omitted from the reaction medium. The phosphorylation of stromal proteins is therefore catalysed by a discrete soluble protein kinase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: inactivation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and activation by phosphorolysis. Arch Biochem Biophys. 1984 May 1;230(2):492–503. doi: 10.1016/0003-9861(84)90429-6. [DOI] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):53–60. doi: 10.1016/0006-291x(83)90967-1. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: identification of a catalytically important histidine residue and its role in the regulation of pyruvate,Pi dikinase. Arch Biochem Biophys. 1984 May 15;231(1):175–182. doi: 10.1016/0003-9861(84)90375-8. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Polya G. M. Purification and properties of a high specific activity protein kinase from wheat germ. Plant Physiol. 1983 Mar;71(3):489–495. doi: 10.1104/pp.71.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C. Phosphorylation of a stromal enzyme protein in maize (Zea mays) mesophyll chloroplasts. Biochem J. 1984 Aug 15;222(1):247–253. doi: 10.1042/bj2220247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Foyer C. Relationships between protein phosphorylation and electron transport in the reconstituted chloroplast system. Biochem J. 1983 Feb 15;210(2):517–521. doi: 10.1042/bj2100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Lin Z. F., Lucero H. A., Racker E. Protein kinases from spinach chloroplasts. I. Purification and identification of two distinct protein kinases. J Biol Chem. 1982 Oct 25;257(20):12153–12156. [PubMed] [Google Scholar]
- Lucero H. A., Lin Z. F., Racker E. Protein kinases from spinach chloroplasts. II. Protein substrate specificity and kinetic properties. J Biol Chem. 1982 Oct 25;257(20):12157–12160. [PubMed] [Google Scholar]
- Polya G. M., Davies J. R. Resolution and properties of a protein kinase catalyzing the phosphorylation of a wheat germ cytokinin-binding protein. Plant Physiol. 1983 Mar;71(3):482–488. doi: 10.1104/pp.71.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Inhibition of spinach phosphoribulokinase by DL-glyceraldehyde. Biochem J. 1976 Mar 1;153(3):613–619. doi: 10.1042/bj1530613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Buchanan B. B. Phosphorylation of chloroplast ribulose bisphosphate carboxylase/oxygenase small subunit by an envelope-bound protein kinase in situ. J Biol Chem. 1983 Jun 10;258(11):6686–6689. [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium- and calmodulin-regulated phosphorylation of soluble and membrane proteins from corn coleoptiles. Plant Physiol. 1984 Oct;76(2):359–365. doi: 10.1104/pp.76.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]




