Abstract
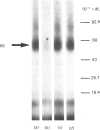
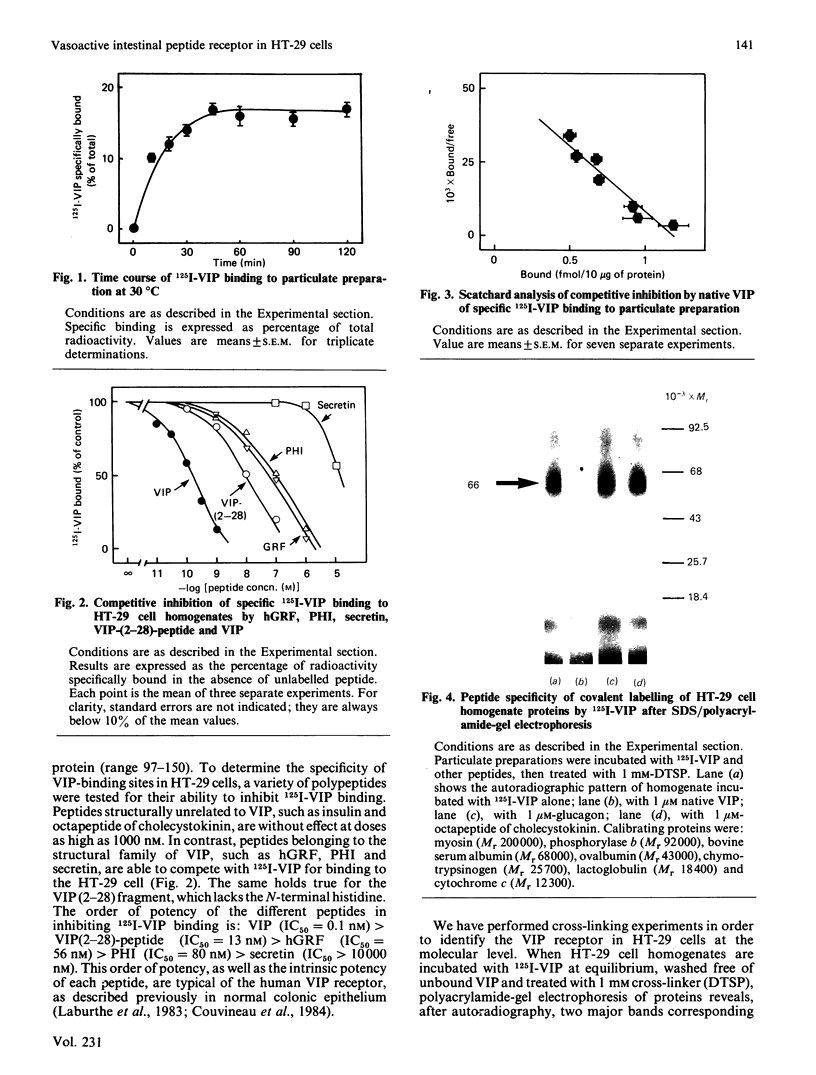
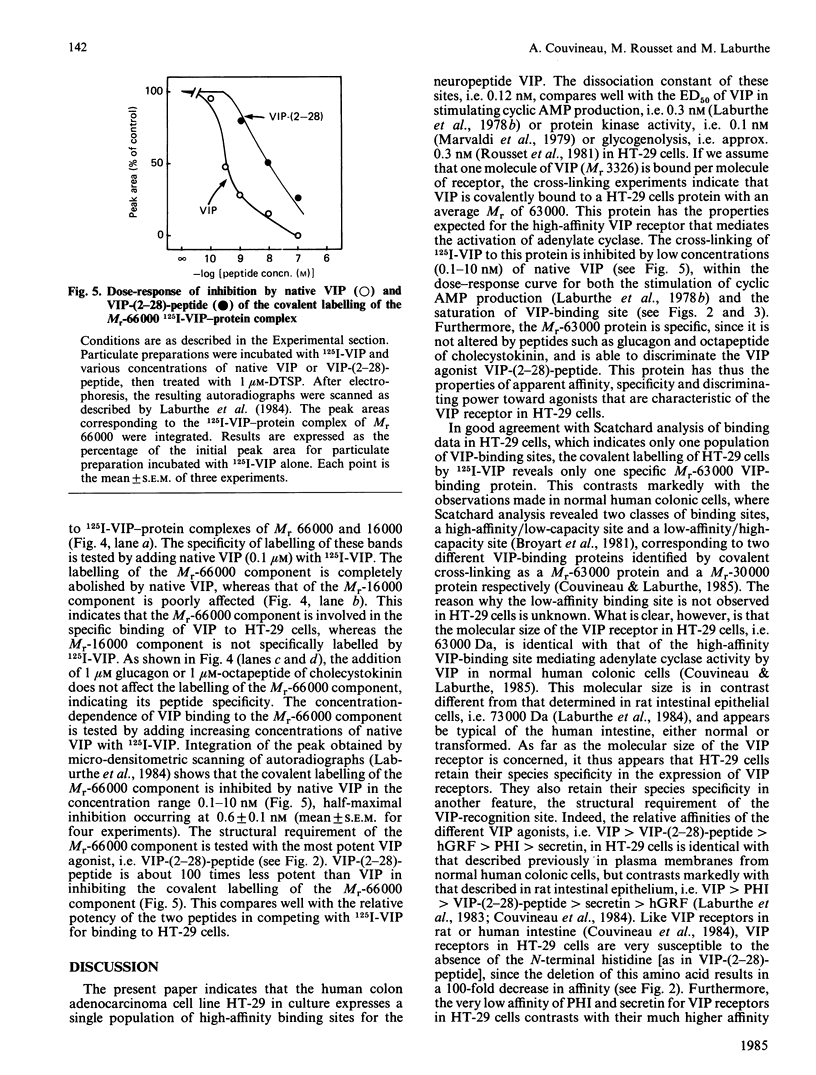
The human colon adenocarcinoma cell line HT-29 in culture exhibits a cyclic AMP production system highly sensitive to vasoactive intestinal peptide (VIP), making HT-29 cells a unique cultured cell system for studying the mechanism of VIP action [Laburthe, Rousset, Boissard, Chevalier, Zweibaum & Rosselin (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 2772-2775]. The quantitative characteristics of VIP receptors in HT-29 cells and their structural requirement and molecular size were studied. 125I-labeled VIP bound in a time-dependent manner to HT-29 cell homogenates. At equilibrium (60 min incubation at 30 degrees C), unlabelled VIP in the 0.01-10 nM concentration range competed with 125I-VIP for binding to cell homogenates. Scatchard analysis of binding data gave a straight line, indicating that VIP bound to a single population of sites with a KD of 0.12 +/- 0.02 nM and a capacity of 120 +/- 9 fmol/mg of protein. The structural requirement of these receptors was studied with peptides structurally related to VIP, either natural or synthetic. Several peptides inhibited 125I-VIP binding to HT-29 cell homogenates with the following order of potency, which is typical of the human VIP receptor: VIP (IC50 = 0.1 nM) greater than VIP-(2-28)-peptide (IC50 = 13 nM) greater than human growth hormone releasing factor (IC50 = 56 nM) greater than peptide histidine isoleucine amide (IC50 = 80 nM) greater than secretin (IC50 greater than 10 000 nM). To characterize the molecular component(s) of the VIP receptor in HT-29 cells, 125I-VIP was covalently bound to cell homogenates by using the cross-linker dithiobis(succinimidyl propionate). Sodium dodecyl sulphate/polyacrylamide-gel autoradiographic studies of affinity-labelled cell homogenates revealed two major bands, corresponding to 125I-VIP-protein complexes of Mr 66 000 and 16 000. The labelling of the Mr-66 000 component was specific, since it was abolished by native VIP, whereas that of the Mr-16 000 component was not. Densitometric scanning of autoradiographs indicated that the labelling of the Mr-66 000 complex was inhibited by low VIP concentrations in the 0.1-10 nM range (IC50 = 0.6 nM), but was unaffected by 1 microM-glucagon or octapeptide of cholecystokinin. It was also decreased by VIP-(2-28)-peptide with a potency 1% that of VIP. Assuming that one molecule of 125I-VIP bound per molecule of protein, one protein of Mr 63 000 was identified as a component of the VIP receptor in HT-29 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiranoff B., Vauclin-Jacques N., Laburthe M. Interaction of gastric inhibitory polypeptide (GIP) with the insulin-secreting pancreatic beta cell line, In lll: characteristics of GIP binding sites. Life Sci. 1985 Mar 4;36(9):807–813. doi: 10.1016/0024-3205(85)90203-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broyart J. P., Dupont C., Laburthe M., Rosselin G. Characterization of vasoactive intestinal peptide receptors in human colonic epithelial cells. J Clin Endocrinol Metab. 1981 Apr;52(4):715–721. doi: 10.1210/jcem-52-4-715. [DOI] [PubMed] [Google Scholar]
- Couvineau A., Laburthe M. The human vasoactive intestinal peptide receptor: molecular identification by covalent cross-linking in colonic epithelium. J Clin Endocrinol Metab. 1985 Jul;61(1):50–55. doi: 10.1210/jcem-61-1-50. [DOI] [PubMed] [Google Scholar]
- Couvineau A., Rouyer-Fessard C., Fournier A., St Pierre S., Pipkorn R., Laburthe M. Structural requirements for VIP interaction with specific receptors in human and rat intestinal membranes: effect of nine partial sequences. Biochem Biophys Res Commun. 1984 Jun 15;121(2):493–498. doi: 10.1016/0006-291x(84)90209-2. [DOI] [PubMed] [Google Scholar]
- Fournier A., Saunders J. K., St-Pierre S. Synthesis, conformational studies and biological activities of VIP and related fragments. Peptides. 1984 Mar-Apr;5(2):169–177. doi: 10.1016/0196-9781(84)90202-x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Laburthe M. C., Dupont C. M., Besson J. D., Rousset M., Rosselin G. E. A new bioassay of VIP: results in watery diarrhoea syndrome. Gut. 1980 Jul;21(7):619–623. doi: 10.1136/gut.21.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M., Amiranoff B., Boige N., Rouyer-Fessard C., Tatemoto K., Moroder L. Interaction of GRF with VIP receptors and stimulation of adenylate cyclase in rat and human intestinal epithelial membranes. Comparison with PHI and secretin. FEBS Lett. 1983 Aug 8;159(1-2):89–92. doi: 10.1016/0014-5793(83)80422-0. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Bataille D., Rosselin G. Vasoactive intestinal peptide (VIP): variation of the jejuno-ileal content in the developing rat as measured by radioreceptorassay. Acta Endocrinol (Copenh) 1977 Mar;84(3):588–599. doi: 10.1530/acta.0.0840588. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Breant B., Rouyer-Fessard C. Molecular identification of receptors for vasoactive intestinal peptide in rat intestinal epithelium by covalent cross-linking. Evidence for two classes of binding sites with different structural and functional properties. Eur J Biochem. 1984 Feb 15;139(1):181–187. doi: 10.1111/j.1432-1033.1984.tb07992.x. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Rosselin G., Rousset M., Zweibaum A., Korner M., Selinger Z., Schramm M. Transfer of the hormone receptor for vaso-intestinal peptide to an adenylate cyclase system in another cell. FEBS Lett. 1979 Feb 1;98(1):41–43. doi: 10.1016/0014-5793(79)80147-7. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Rousset M., Boissard C., Chevalier G., Zweibaum A., Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3':5'-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2772–2775. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M., Rousset M., Chevalier G., Boissard C., Dupont C., Zweibaum A., Rosselin G. Vasoactive intestinal peptide control of cyclic adenosine 3':5'-monophosphate levels in seven human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1980 Jul;40(7):2529–2533. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marvaldi J., Mangeat P., Ahmed O. A., Coeroli C., Marchis-Mouren G. Activation of cyclic AMP-dependent protein kinases in human gut adenocarcinoma (HT 29) cells in culture. Biochim Biophys Acta. 1979 Nov 15;588(1):12–19. doi: 10.1016/0304-4165(79)90365-9. [DOI] [PubMed] [Google Scholar]
- Pichon J., Hirn M., Muller J. M., Mangeat P., Marvaldi J. Anti-cell surface monoclonal antibodies which antagonize the action of VIP in a human adenocarcinoma cell line (HT 29 cells). EMBO J. 1983;2(7):1017–1022. doi: 10.1002/j.1460-2075.1983.tb01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset M., Laburthe M., Chevalier G., Boissard C., Rosselin G., Zweibaum A. Vasoactive intestinal peptide (VIP) control of glycogenolysis in the human colon carcinoma cell line HT-29 in culture. FEBS Lett. 1981 Apr 6;126(1):38–40. doi: 10.1016/0014-5793(81)81027-7. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980 Jun 5;285(5764):417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]