Abstract
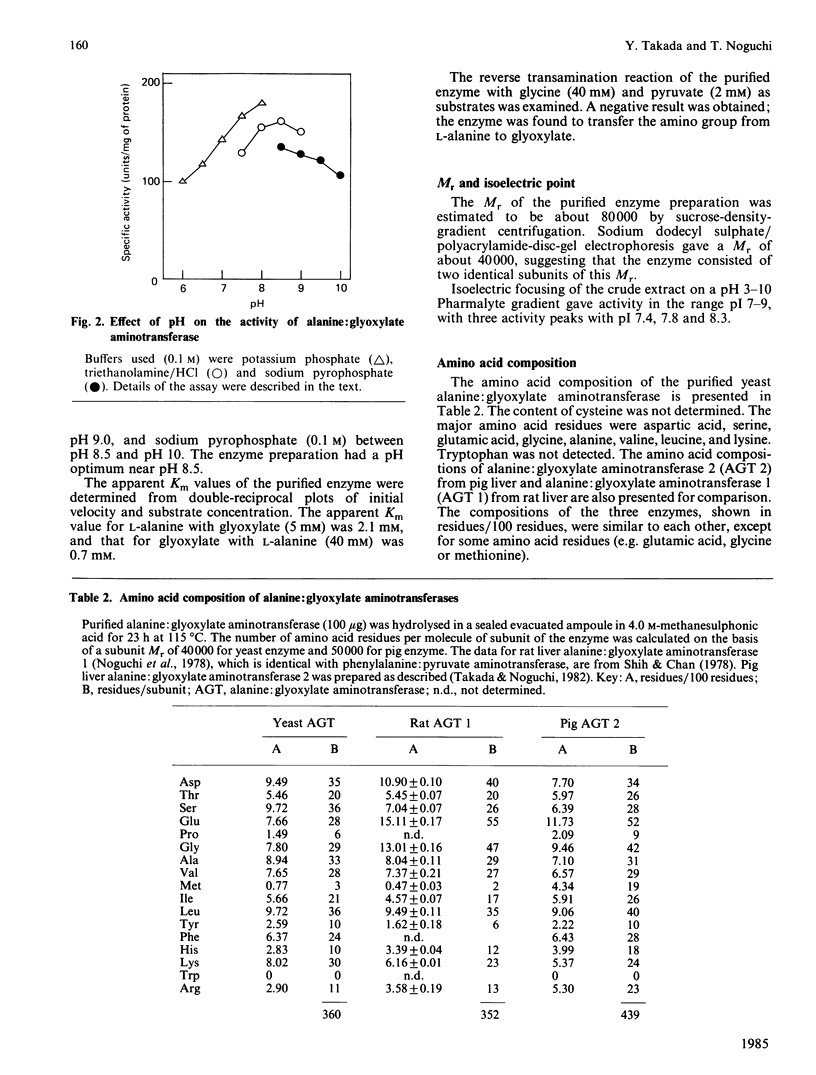
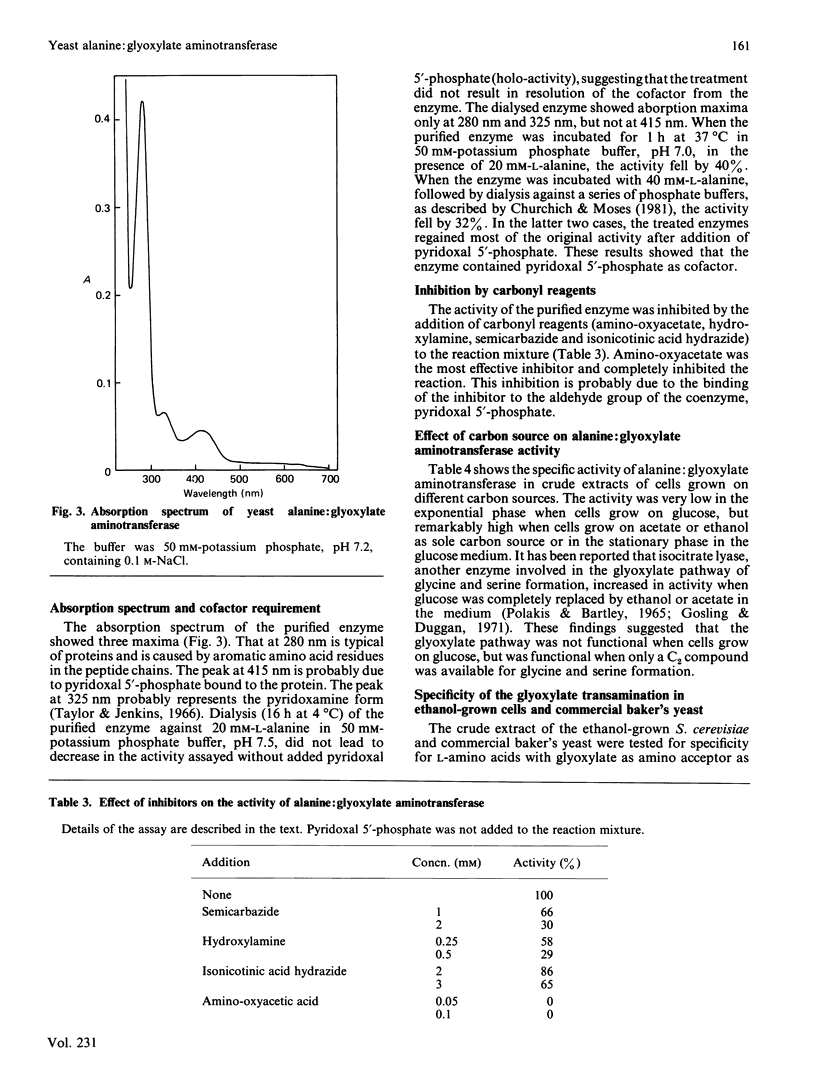
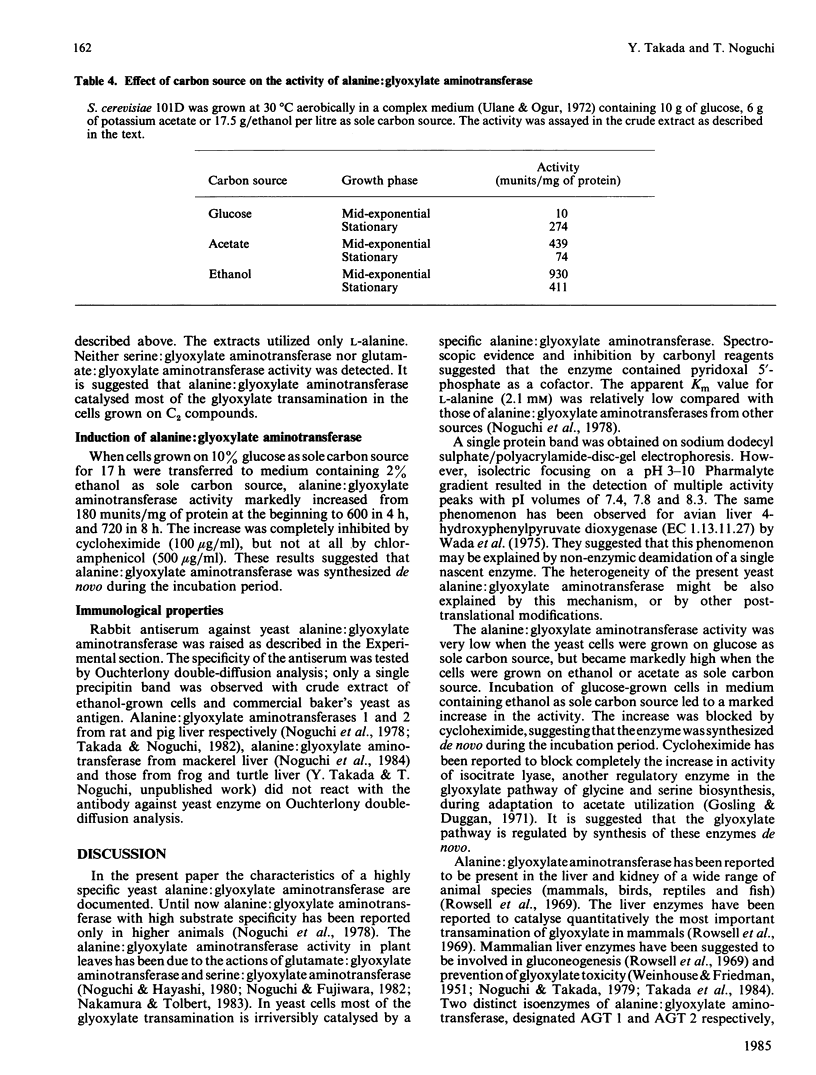
Alanine: glyoxylate aminotransferase (EC 2.6.1.44), which is involved in the glyoxylate pathway of glycine and serine biosynthesis from tricarboxylic acid-cycle intermediates in Saccharomyces cerevisiae, was highly purified and characterized. The enzyme had Mr about 80 000, with two identical subunits. It was highly specific for L-alanine and glyoxylate and contained pyridoxal 5'-phosphate as cofactor. The apparent Km values were 2.1 mM and 0.7 mM for L-alanine and glyoxylate respectively. The activity was low (10 nmol/min per mg of protein) with glucose as sole carbon source, but was remarkably high with ethanol or acetate as carbon source (930 and 430 nmol/min per mg respectively). The transamination of glyoxylate is mainly catalysed by this enzyme in ethanol-grown cells. When glucose-grown cells were incubated in medium containing ethanol as sole carbon source, the activity markedly increased, and the increase was completely blocked by cycloheximide, suggesting that the enzyme is synthesized de novo during the incubation period. Similarity in the amino acid composition was observed, but immunological cross-reactivity was not observed among alanine: glyoxylate aminotransferases from yeast and vertebrate liver.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Churchich J. E., Moses U. 4-Aminobutyrate aminotransferase. The presence of nonequivalent binding sites. J Biol Chem. 1981 Feb 10;256(3):1101–1104. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Boiso J. F., Stoppani A. O. Metabolism of serine and glycine in baker's yeast. Biochim Biophys Acta. 1967 Oct 9;148(1):48–59. doi: 10.1016/0304-4165(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Gosling J. P., Duggan P. F. Activities of tricarboxylic acid cycle enzymes, glyoxylate cycle enzymes, and fructose diphosphatase in bakers' yeast during adaptation to acetate oxidation. J Bacteriol. 1971 Jun;106(3):908–914. doi: 10.1128/jb.106.3.908-914.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nakamura Y., Tolbert N. E. Serine: glyoxylate, alanine:glyoxylate, and glutamate:glyoxylate aminotransferase reactions in peroxisomes from spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7631–7638. [PubMed] [Google Scholar]
- Noguchi T., Fujiwara S. Development of glutamate:glyoxylate aminotransferase in the cotyledons of cucumber (Cucumis sativus) seedlings. Biochem J. 1982 Jan 1;201(1):209–214. doi: 10.1042/bj2010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Fujiwara S., Takada Y., Mori T., Nagano M., Hanada N., Saeki E., Yasuo O. Enzymatic and immunological comparison of alanine: glyoxylate aminotransferases from different fish and mammalian livers. Comp Biochem Physiol B. 1984;77(2):279–283. doi: 10.1016/0305-0491(84)90330-4. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Hayashi S. Peroxisomal localization and properties of tryptophan aminotransferase in plant leaves. J Biol Chem. 1980 Mar 25;255(6):2267–2269. [PubMed] [Google Scholar]
- Noguchi T., Okuno E., Takada Y., Minatogawa Y., Okai K., Kido R. Characteristics of hepatic alanine-glyoxylate aminotransferase in different mammalian species. Biochem J. 1978 Jan 1;169(1):113–122. doi: 10.1042/bj1690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Takada Y. Peroxisomal localization of alanine: glyoxylate aminotransferase in human liver. Arch Biochem Biophys. 1979 Sep;196(2):645–647. doi: 10.1016/0003-9861(79)90319-9. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Al-Tai A. H. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969 Dec;115(5):1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschlau P., Hess B. Purification and crystallization of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1972 Mar;353(3):435–440. doi: 10.1515/bchm2.1972.353.1.435. [DOI] [PubMed] [Google Scholar]
- Shih J. C., Chan Y. L. Purification and properties of two forms of hepatic phenylalanine:pyruvate transaminase from glucagon treated rats. Arch Biochem Biophys. 1978 Aug;189(2):454–464. doi: 10.1016/0003-9861(78)90234-5. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Takada Y., Mori T., Noguchi T. The effect of vitamin B6 deficiency on alanine: glyoxylate aminotransferase isoenzymes in rat liver. Arch Biochem Biophys. 1984 Feb 15;229(1):1–6. doi: 10.1016/0003-9861(84)90123-1. [DOI] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. Subcellular distribution, and physical and immunological properties of hepatic alanine: glyoxylate aminotransferase isoenzymes in different mammalian species. Comp Biochem Physiol B. 1982;72(4):597–604. doi: 10.1016/0305-0491(82)90512-0. [DOI] [PubMed] [Google Scholar]
- Taylor R. T., Jenkins W. T. Leucine aminotransferase. II. Purification and characterization. J Biol Chem. 1966 Oct 10;241(19):4396–4405. [PubMed] [Google Scholar]
- WEINHOUSE S., FRIEDMANN B. Metabolism of labeled 2-carbon acids in the intact rat. J Biol Chem. 1951 Aug;191(2):707–717. [PubMed] [Google Scholar]
- Wada G. H., Fellman J. H., Fujita T. S., Roth E. S. Purification and properties of avian liver p-hydroxyphenylpyruvate hydroxylase. J Biol Chem. 1975 Sep 10;250(17):6720–6726. [PubMed] [Google Scholar]