Abstract
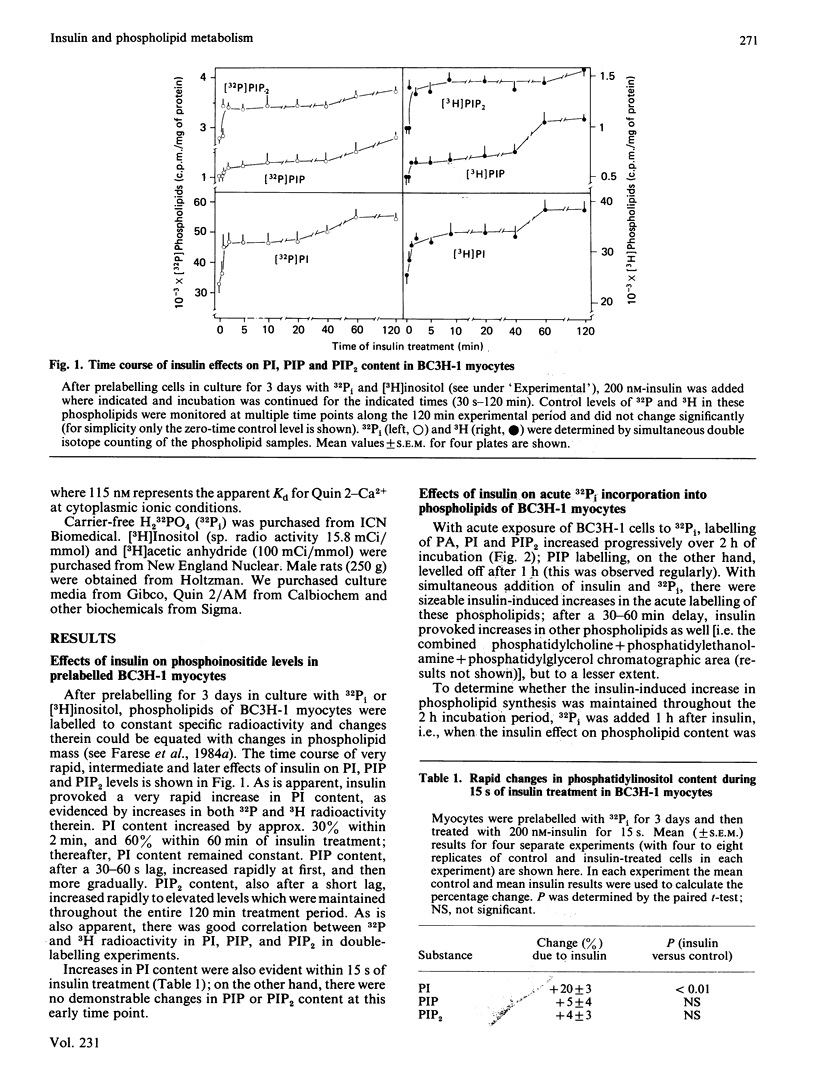
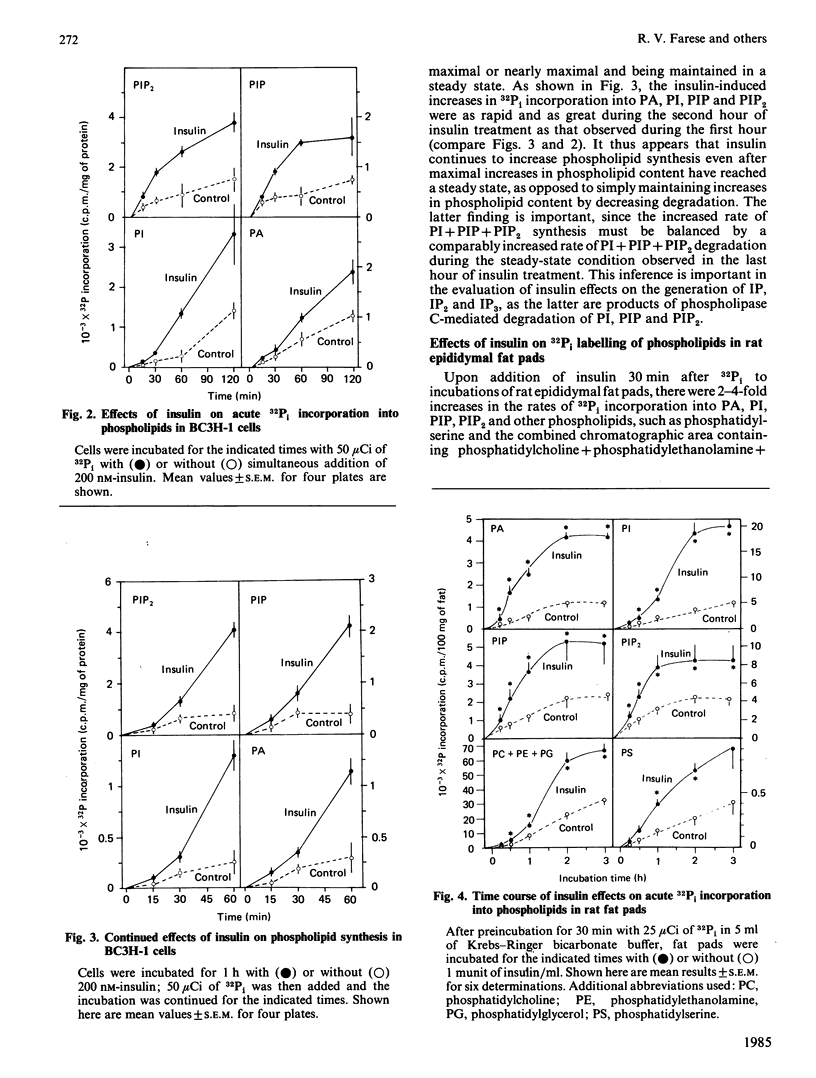
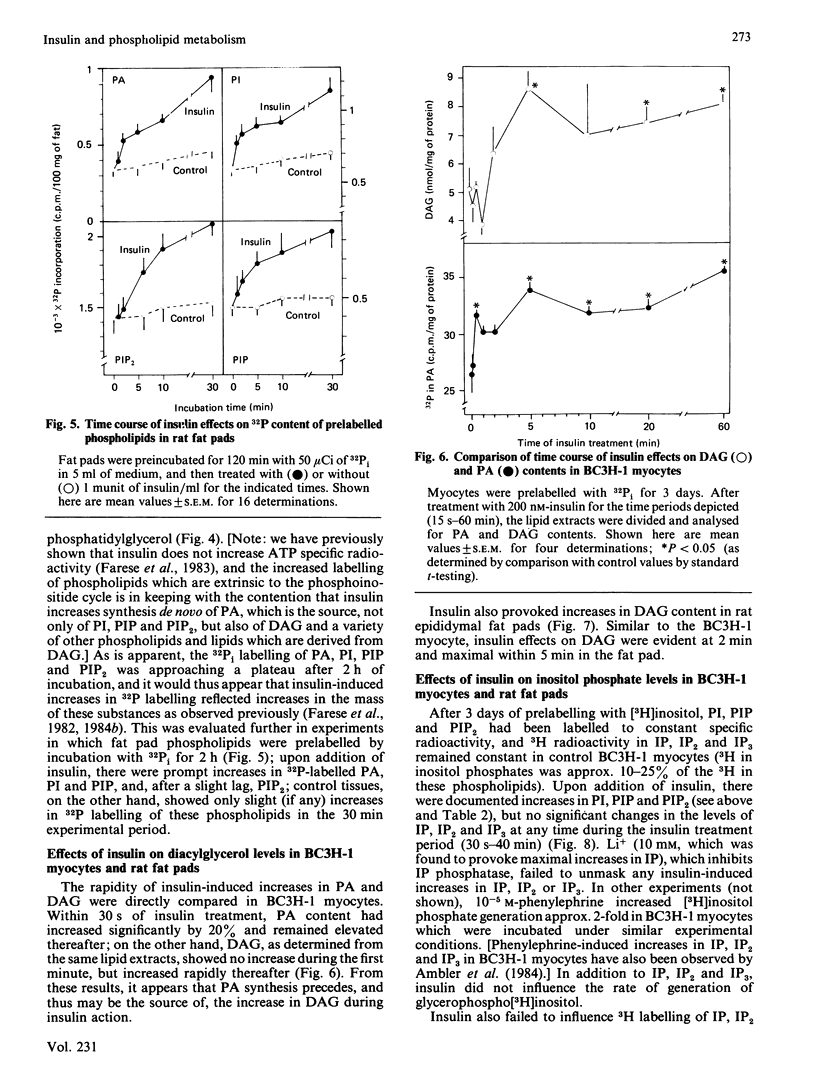
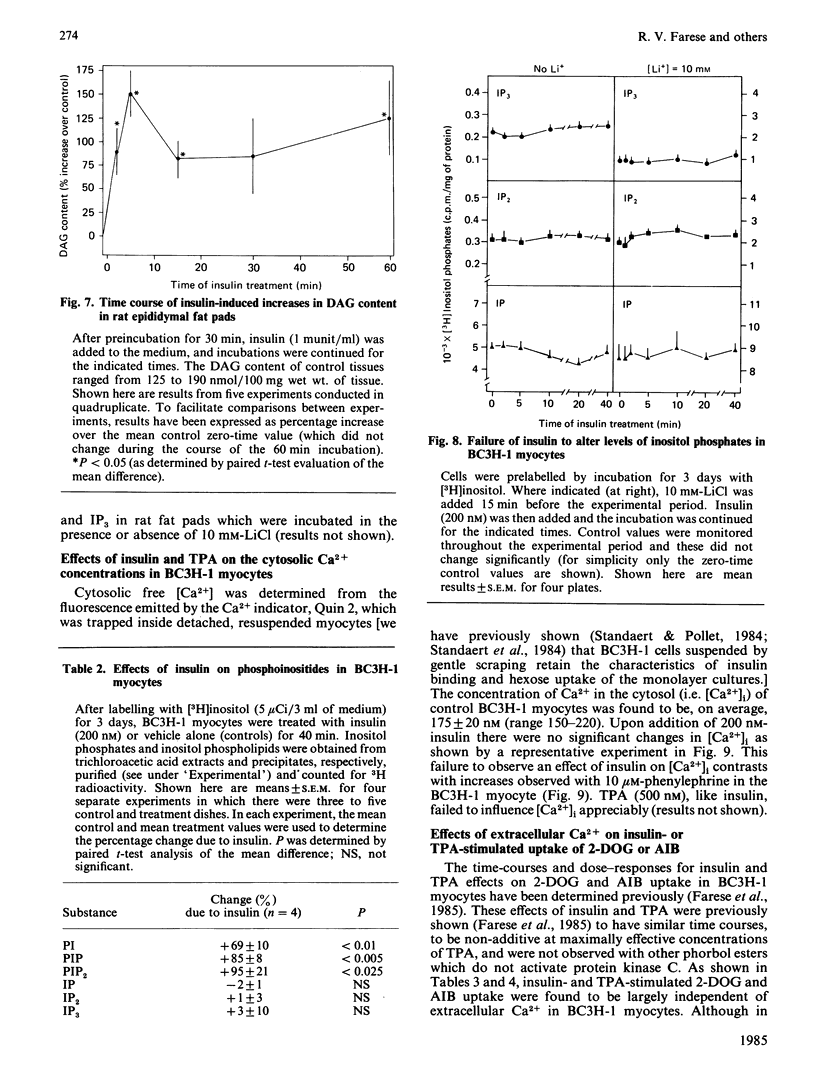
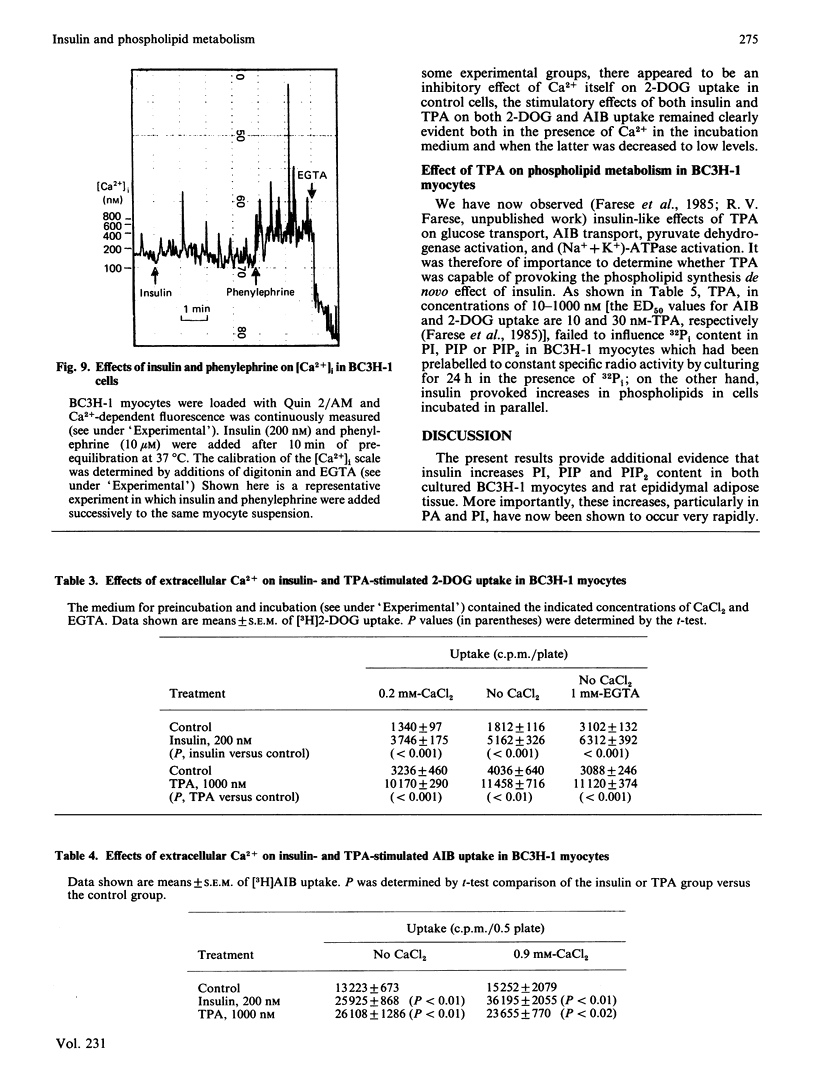
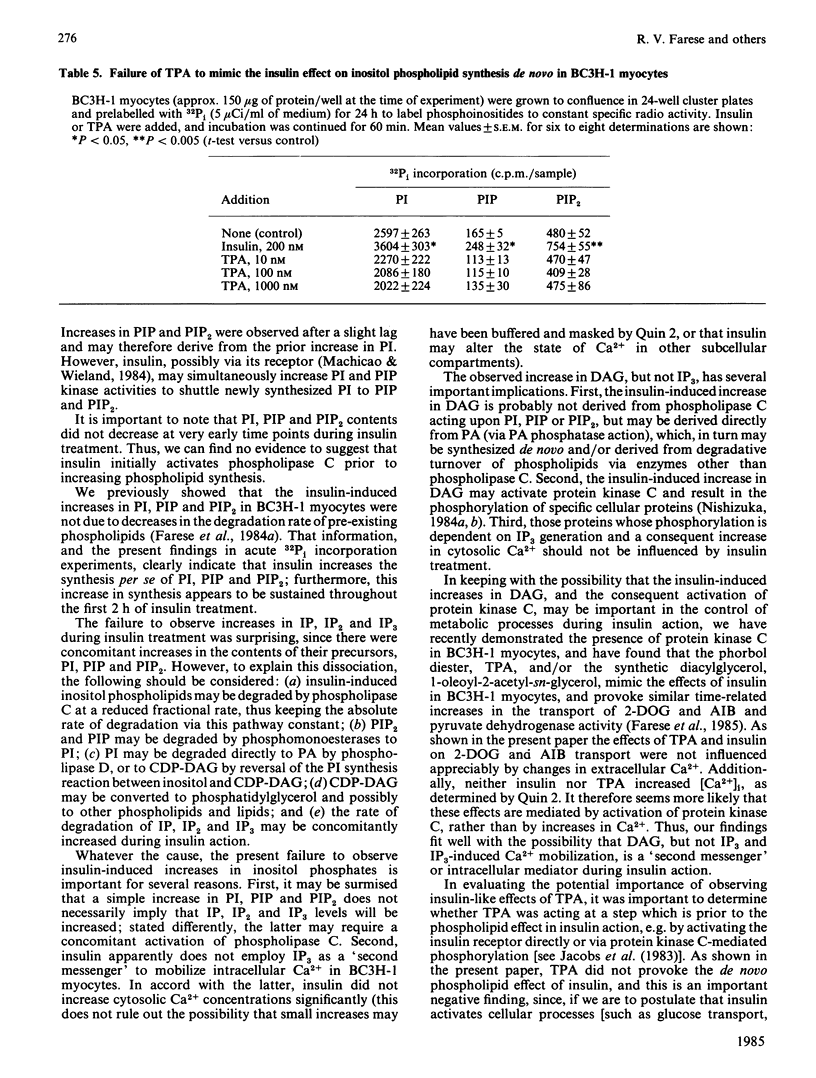
We have previously reported that insulin increases the synthesis de novo of phosphatidic acid (PA), phosphatidylinositol (PI), phosphatidylinositol 4-phosphate (PIP), phosphatidylinositol 4,5-bisphosphate (PIP2) and diacylglycerol (DAG) in BC3H-1 myocytes and/or rat adipose tissue. Here we have further characterized these effects of insulin and examined whether there are concomitant changes in inositol phosphate generation and Ca2+ mobilization. We found that insulin provoked very rapid increases in PI content (20% within 15 s in myocytes) and, after a slight lag, PIP and PIP2 content in both BC3H-1 myocytes and rat fat pads (measured by increases in 32P or 3H content after prelabelling phospholipids to constant specific radioactivity by prior incubation with 32Pi or [3H]inositol). Insulin also increased 32Pi incorporation into these phospholipids when 32Pi was added either simultaneously with insulin or 1 h after insulin. Thus, the insulin-induced increase in phospholipid content appeared to be due to an increase in phospholipid synthesis, which was maintained for at least 2 h. Insulin increased DAG content in BC3H-1 myocytes and adipose tissue, but failed to increase the levels of inositol monophosphate (IP), inositol bisphosphate (IP2) or inositol trisphosphate (IP3). The failure to observe an increase in IP3 (a postulated 'second messenger' which mobilizes intracellular Ca2+) was paralleled by a failure to observe an insulin-induced increase in the cytosolic concentration of Ca2+ in BC3H-1 myocytes as measured by Quin 2 fluorescence. Like insulin, the phorbol diester 12-O-tetradecanoylphorbol 13-acetate (TPA) increased the transport of 2-deoxyglucose and aminoisobutyric acid in BC3H-1 myocytes. These effects of insulin and TPA appeared to be independent of extracellular Ca2+. We conclude that the phospholipid synthesis de novo effect of insulin is provoked very rapidly, and is attended by increases in DAG but not IP3 or Ca2+ mobilization. The insulin-induced increase in DAG does not appear to be a consequence of phospholipase C acting upon the expanded PI + PIP + PIP2 pool, but may be derived directly from PA. Our findings suggest the possibility that DAG (through protein kinase C activation) may function as an important intracellular 'messenger' for controlling metabolic processes during insulin action.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Billah M. M., Finean J. B., Michell R. H. Release of diacylglycerol-enriched vesicles from erythrocytes with increased intracellular (Ca2+). Nature. 1976 May 6;261(5555):58–60. doi: 10.1038/261058a0. [DOI] [PubMed] [Google Scholar]
- Ambler S. K., Brown R. D., Taylor P. The relationship between phosphatidylinositol metabolism and mobilization of intracellular calcium elicited by alpha1-adrenergic receptor stimulation in BC3H-1 muscle cells. Mol Pharmacol. 1984 Nov;26(3):405–413. [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., Hokin-Neaverson M. Acetylcholine increases the level of diglyceride in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):714–718. doi: 10.1016/s0006-291x(74)80476-6. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Topping D. L., Sooranna S. P., Saggerson D., Mayes P. A. Acute effects of insulin on glycerol phosphate acyl transferase activity, ketogenesis and serum free fatty acid concentration in perfused rat liver. FEBS Lett. 1977 Dec 15;84(2):225–228. doi: 10.1016/0014-5793(77)80693-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. 5-Hydroxytryptamine stimulation of phosphatidylinositol hydrolysis and calcium signalling in the blowfly salivary gland. Cell Calcium. 1982 Oct;3(4-5):385–397. doi: 10.1016/0143-4160(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Vaartjes W. J., Geelen M. J. Acute effects of insulin on fatty acid metabolism in isolated rat hepatocytes. Horm Metab Res. 1980 Sep;12(9):425–430. doi: 10.1055/s-2007-999166. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Insulin action. Am J Med. 1981 Jan;70(1):142–150. doi: 10.1016/0002-9343(81)90421-6. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Barnes D. E., Davis J. S., Standaert M. L., Pollet R. J. Effects of insulin and protein synthesis inhibitors on phospholipid metabolism, diacylglycerol levels, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. J Biol Chem. 1984 Jun 10;259(11):7094–7100. [PubMed] [Google Scholar]
- Farese R. V., Farese R. V., Jr, Sabir M. A., Larson R. E., Trudeau W. L., 3rd, Barnes D. The mechanism of action of insulin on phospholipid metabolism in rat adipose tissue. Requirement for protein synthesis and a carbohydrate source, and relationship to activation of pyruvate dehydrogenase. Diabetes. 1984 Jul;33(7):648–655. doi: 10.2337/diab.33.7.648. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Insulin acutely increases phospholipids in the phosphatidate-inositide cycle in rat adipose tissue. J Biol Chem. 1982 Apr 25;257(8):4042–4045. [PubMed] [Google Scholar]
- Farese R. V., Sabir M. A., Larson R. E., Trudeau W., 3rd Further observations on the increases in inositide phospholipids after stimulation by ACTH, cAMP and insulin, and on discrepancies in phosphatidylinositol mass and 32PO4-labeling during inhibition of hormonal effects by cycloheximide. Cell Calcium. 1983 Jul;4(3):195–218. doi: 10.1016/0143-4160(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Barnes D. E., Davis J. S., Pollet R. J. Phorbol ester provokes insulin-like effects on glucose transport, amino acid uptake, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. Endocrinology. 1985 Jun;116(6):2650–2655. doi: 10.1210/endo-116-6-2650. [DOI] [PubMed] [Google Scholar]
- Geelen M. J., Groener J. E., de Haas C. G., Wisserhof T. A., van Golde L. M. Influence of insulin and glucagon on the synthesis of glycerolipids in rat hepatocytes. FEBS Lett. 1978 Jun 1;90(1):57–60. doi: 10.1016/0014-5793(78)80297-x. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kimura S., Nagasaki K., Adachi I., Yamaguchi K., Fujiki H., Abe K. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoylphorbol-13-acetate (TPA) via a calcium requiring process. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1057–1064. doi: 10.1016/0006-291x(84)91198-7. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Michell R. H., Hems D. A. Phosphatidylinositol metabolism in rat hepatocytes stimulated by vasopressin. Biochem J. 1981 Jan 15;194(1):155–165. doi: 10.1042/bj1940155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machicao E., Wieland O. H. Evidence that the insulin receptor-associated protein kinase acts as a phosphatidylinositol kinase. FEBS Lett. 1984 Sep 17;175(1):113–116. doi: 10.1016/0014-5793(84)80581-5. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Roach P. J., Goldman M. Modification of glycogen synthase activity in isolated rat hepatocytes by tumor-promoting phorbol esters: evidence for differential regulation of glycogen synthase and phosphorylase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7170–7172. doi: 10.1073/pnas.80.23.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert M. L., Pollet R. J. Equilibrium model for insulin-induced receptor down-regulation. Regulation of insulin receptors in differentiated BC3H-1 myocytes. J Biol Chem. 1984 Feb 25;259(4):2346–2354. [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D., Uhing R. J., Blackmore P. F., Prpić V., Exton J. H. Insulin and epidermal growth factor do not affect phosphoinositide metabolism in rat liver plasma membranes and hepatocytes. J Biol Chem. 1985 Feb 25;260(4):2011–2014. [PubMed] [Google Scholar]
- Trevillyan J. M., Perisic O., Traugh J. A., Byus C. V. Insulin- and phorbol ester-stimulated phosphorylation of ribosomal protein S6. J Biol Chem. 1985 Mar 10;260(5):3041–3044. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]


