Abstract
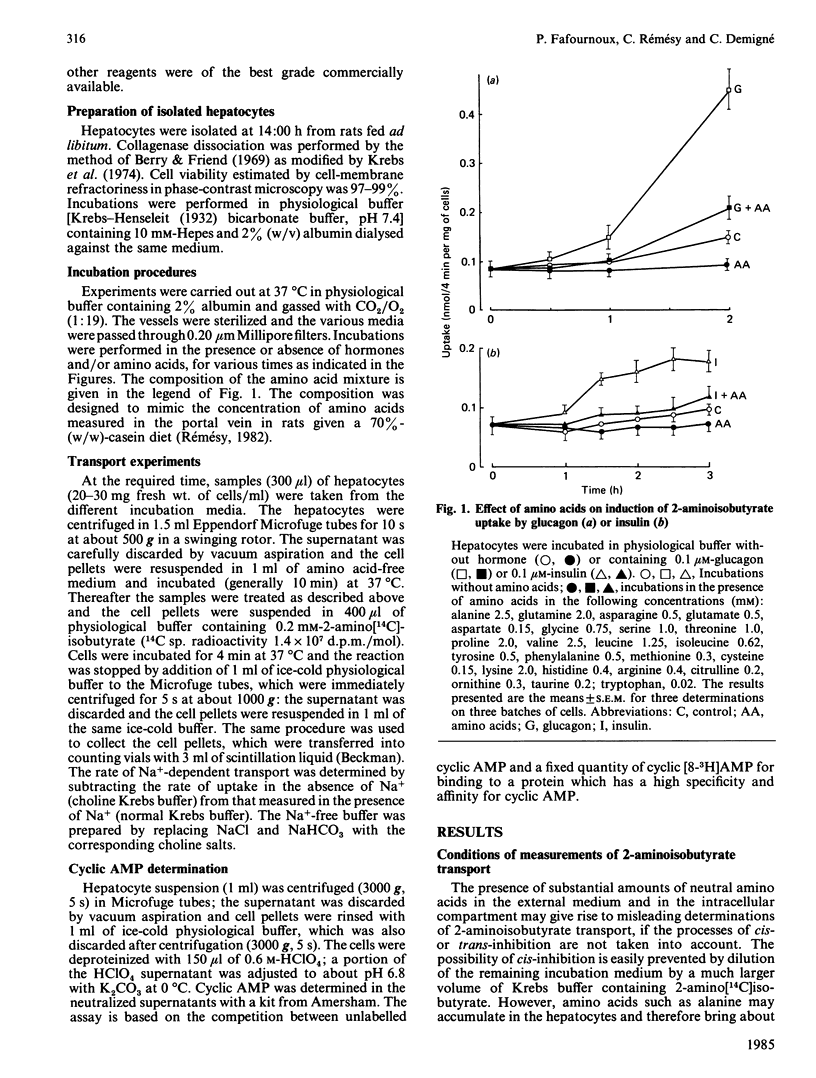
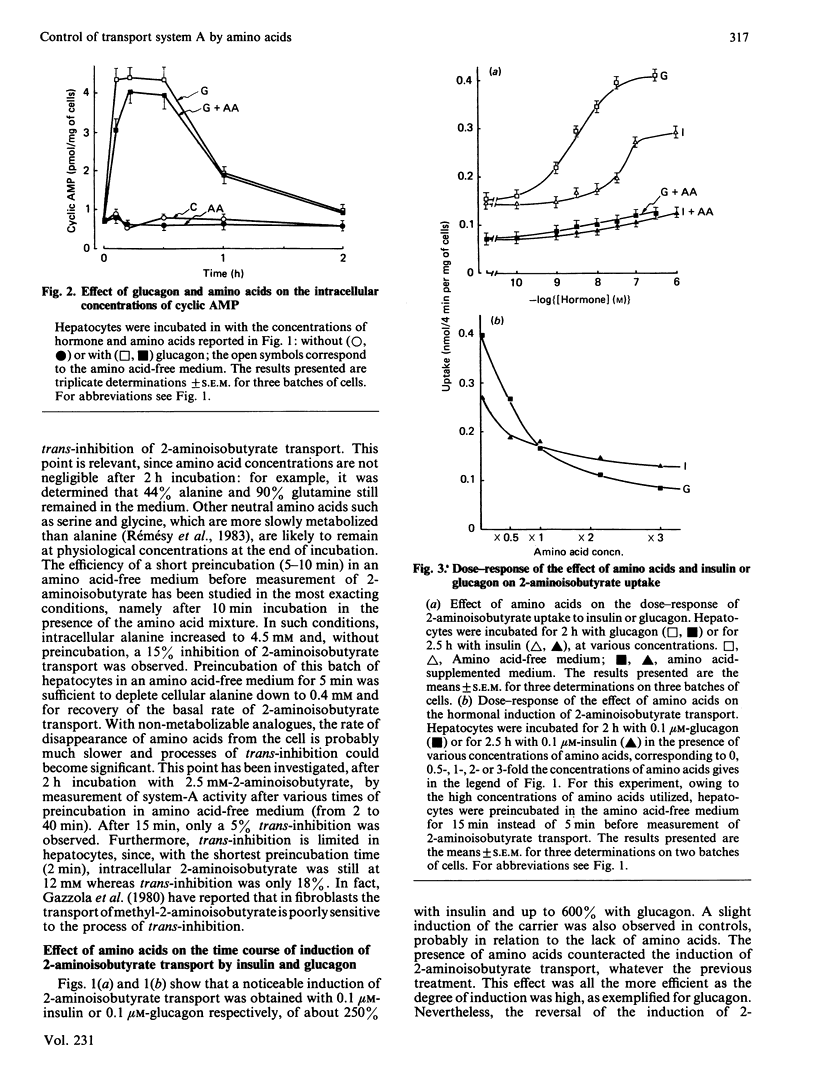
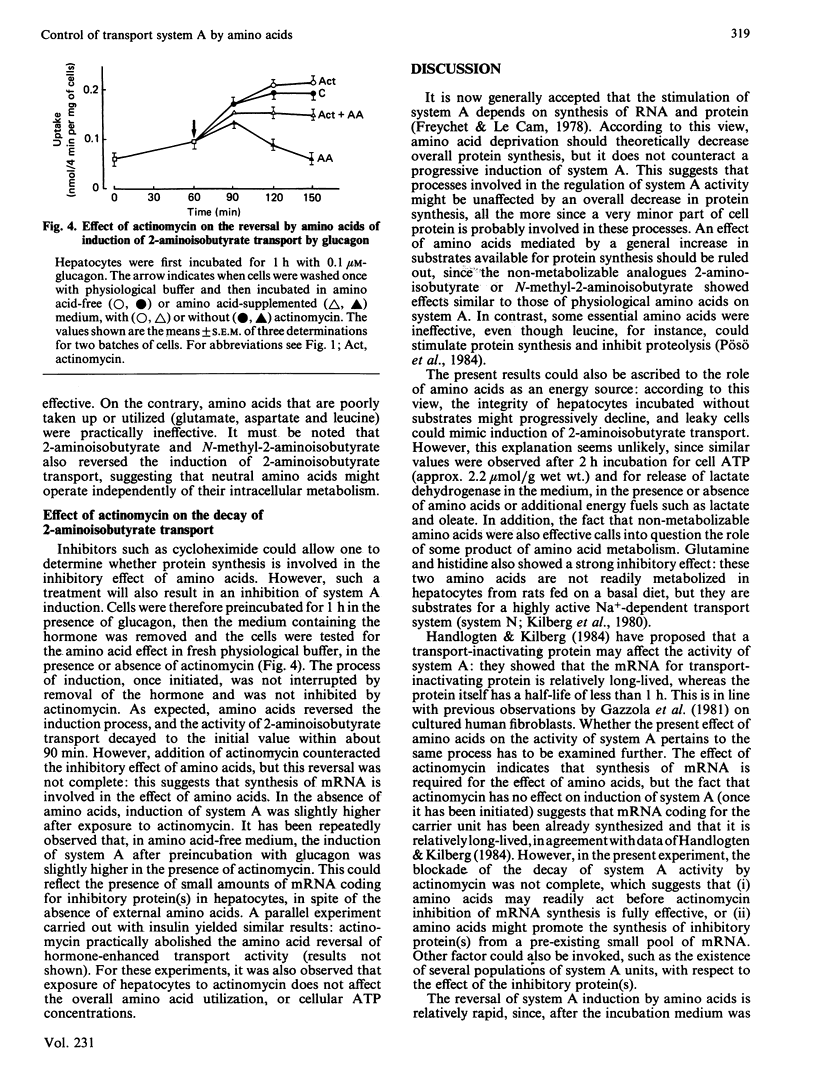
The effect of amino acids, in concentrations corresponding to those found in the portal vein of rats given a high-protein diet, was investigated on the activity of system A amino acid transport in hepatocytes from fed rats. Amino acids counteracted the induction of system A by insulin or glucagon. This effect was observed at all concentrations of hormones tested, up to 1 microM. Amino acids did not affect the basal cyclic AMP concentration in hepatocytes, or the large rise in cyclic AMP elicited by glucagon. The reversal of system-A induction was observed at relatively low concentration of amino acids, corresponding to plasma values reported in rats given a basal diet. Amino acids were separately tested: substrates of system A were particularly efficient, but so were glutamine and histidine. Non-metabolizable substrates of system A, such as 2-aminoisobutyrate, were also inhibitory, suggesting that a part of the effect of amino acids is independent of their cellular metabolism. Provision of additional energy substrates such as lactate and oleate did not affect induction of system A or the inhibitory effects of amino acids. Thus amino acids do not act by serving as an energy source and by maintaining the integrity of hepatocytes. Inhibition of mRNA synthesis by actinomycin practically abolished the effect of amino acids on the induction of system A by glucagon. The results suggest that amino acids may promote the synthesis of protein(s) affecting the activity of system A either directly at the carrier unit or at an intermediate stage of its emergence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Grunwald T. Potassium permeability and volume control in isolated rat hepatocytes. Biochim Biophys Acta. 1983 Jun 10;731(2):239–242. doi: 10.1016/0005-2736(83)90014-7. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafournoux P., Demigné C., Rémésy C. Effect of alanine transport on ionic fluxes across the cell membrane in rat liver. Arch Int Physiol Biochim. 1984 Dec;92(5):369–378. doi: 10.3109/13813458409080613. [DOI] [PubMed] [Google Scholar]
- Fafournoux P., Rémésy C., Demigné C. Control of alanine metabolism in rat liver by transport processes or cellular metabolism. Biochem J. 1983 Mar 15;210(3):645–652. doi: 10.1042/bj2100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafournoux P., Rémésy C., Demigné C. Stimulation of amino acid transport into liver cells from rats adapted to a high-protein diet. Biochem J. 1982 Jul 15;206(1):13–18. doi: 10.1042/bj2060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlmann M., Le Cam A., Freychet P. Insulin and glucagon stimulation of amino acid transport in isolated rat hepatocytes. Synthesis of a high affinity component of transport. J Biol Chem. 1979 Oct 25;254(20):10431–10437. [PubMed] [Google Scholar]
- Fehlmann M., Le Cam A., Kitabgi P., Rey J. F., Freychet P. Regulation of amino acid transport in the liver. Emergence of a high affinity transport system in isolated hepatocytes from fasting rats. J Biol Chem. 1979 Jan 25;254(2):401–407. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3191–3198. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. The transport of neutral amino acids in cultured human fibroblasts. J Biol Chem. 1980 Feb 10;255(3):929–936. [PubMed] [Google Scholar]
- Goldberg M. L., Biava C. G. The effects of glucose and cyclic GMP on RNA synthesis and nuclear morphology in starved rats. Biochim Biophys Acta. 1976 Dec 13;454(3):457–468. doi: 10.1016/0005-2787(76)90272-0. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Handlogten M. E., Kilberg M. S. Induction and decay of amino acid transport in the liver. Turnover of transport activity in isolated hepatocytes after stimulation by diabetes or glucagon. J Biol Chem. 1984 Mar 25;259(6):3519–3525. [PubMed] [Google Scholar]
- Kelley D. S., Evanson T., Potter V. R. Calcium-dependent hormonal regulation of amino acid transport and cyclic AMP accumulation in rat hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5953–5957. doi: 10.1073/pnas.77.10.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [PubMed] [Google Scholar]
- Kristensen L. O., Folke M. Volume-regulatory K+ efflux during concentrative uptake of alanine in isolated rat hepatocytes. Biochem J. 1984 Jul 1;221(1):265–268. doi: 10.1042/bj2210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D., Ramsell J. C., Lacey J. H. Stimulation of alanine transport and metabolism by dibutyryl cyclic AMP in the hepatocytes from fed rats. Assessment of transport as a potential rate-limiting step for alanine metabolism. Biochim Biophys Acta. 1981 Jun 22;644(2):295–304. doi: 10.1016/0005-2736(81)90387-4. [DOI] [PubMed] [Google Scholar]
- Pariza M. W., Butcher F. R., Kletzien R. F., Becker J. E., Potter V. R. Induction and decay of glucagon-induced amino acid transport in primary cultures of adult rat liver cells: paradoxical effects of cycloheximide and puromycin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4511–4515. doi: 10.1073/pnas.73.12.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö A. R., Wert J. J., Jr, Mortimore G. E. Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. J Biol Chem. 1982 Oct 25;257(20):12114–12120. [PubMed] [Google Scholar]
- Remesy C., Fafournoux P., Demigne C. Control of hepatic utilization of serine, glycine and threonine in fed and starved rats. J Nutr. 1983 Jan;113(1):28–39. doi: 10.1093/jn/113.1.28. [DOI] [PubMed] [Google Scholar]
- Samson M., Fehlmann M., Dolais-Kitabgi J., Freychet P. Amino acid transport in isolated hepatocytes from streptozotocin-diabetic rats. Diabetes. 1980 Dec;29(12):996–1000. doi: 10.2337/diab.29.12.996. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Groen A. K., Tager J. M. Plasma-membrane transport of alanine is rate-limiting for its metabolism in rat-liver parenchymal cells. FEBS Lett. 1980 Oct 6;119(2):271–274. doi: 10.1016/0014-5793(80)80269-9. [DOI] [PubMed] [Google Scholar]


