Abstract
Introduction
Infective endocarditis (IE) is a disease that may frequently lead to significant morbidity and is associated with high mortality rates. Even though IE is classically caused by Gram-positive bacteria, Gram-negative bacteria may seldom cause IE. Antimicrobial resistance (AMR) may pose significant problems in treating IE, especially for carbapenem-resistant pathogens. This study aimed to review all cases of IE by carbapenem-resistant Gram-negative bacteria in a systematic way and present information on epidemiology, clinical findings, treatment, and outcomes.
Methods
A systematic review of PubMed, Cochrane Library, and Scopus (all published studies up to 6 August 2023) for published studies providing information on epidemiology, clinical findings, treatment, and outcomes of IE by carbapenem-resistant Gram-negative bacteria was performed.
Results
A total of 24 studies containing data from 26 patients were included. Among all patients, 53.9% were male, and the median age was 66 years. Among all patients, 38.5% had a history of a prosthetic valve. The most commonly affected valve was the aortic, followed by the mitral valve. Fever, sepsis, emboli, and shock were the most frequent clinical findings. The most commonly isolated pathogens were Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii. Aminoglycosides, colistin, cephalosporins, and carbapenems were the most commonly used antimicrobials. Surgery was performed in 53.8% of patients. Mortality was 38.5%.
Conclusions
The development of infection control measures and antimicrobial stewardship interventions is needed to reduce the spread of AMR and the likelihood of this fatal infection.
Keywords: Infective endocarditis, carbapenem-resistant, multidrug resistant, extensively-drug resistant, Pseudomonas, Klebsiella
Introduction
Infective endocarditis (IE) is an infection of the endocardium, most commonly on the cardiac valves or a cardiovascular implantable electronic device (CIED) such as a pacemaker or an implantable cardiac defibrillator, and is associated with high mortality and morbidity rates.1,2 In a relatively recent study, the hospital mortality for patients hospitalized with IE was 17%.3 In another study, the 30-day and the one-year mortality in patients with IE were 14% and 30% respectively.4 Even though Gram-positive bacteria, such as staphylococci, streptococci, and enterococci, are the most commonly isolated microorganisms in IE, adding up to 75% of isolated microorganisms, cases of Gram-negative microorganisms are occasionally reported.5,6
Antimicrobial resistance (AMR) is an emerging global threat, causing millions of deaths each year.7 For example, about five million deaths were associated with AMR in 2019. Most of these deaths are associated with Gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae.7 Carbapenems had been traditionally considered important antimicrobials in the fight against bacteria with AMR. However, the development of carbapenem resistance is an emerging problem that significantly limits the therapeutic options in these patients. Carbapenem resistance can be associated with increased mortality rates.8,9 More specifically, some pathogens, such as A. baumannii, can have multiple mechanisms of AMR that may make the pathogen resistant to most or even all antimicrobials.10
This study aimed to review all cases of IE by carbapenem-resistant Gram-negative bacteria in a systematic way and describe the epidemiology, clinical findings, treatment, and outcomes.
Methods
Data search
For the conduction of the present systematic review, we followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines, as they are more appropriate for systematic reviews assessing epidemiological studies.11 PubMed, Cochrane Library and Scopus, were searched to identify eligible studies by using the text words: ‘(carbapenem OR meropenem OR imipenem OR ertapenem OR doripenem OR biapenem OR tebipenem OR panipenem) AND resist* AND endocarditis’. All studies published until 6 August 2023 were included in further analysis if eligible.
Study selection
The following criteria were required for inclusion of a study in the analysis: 1) Article published in English language; 2) Reporting information on microbiology, clinical characteristics, treatment, and outcomes. Exclusion criteria were the following: 1) Secondary research papers (such as reviews), editorials and any article not providing original information on the subject; 2) Studies not referring to humans; 3) Studies not published in the English language, 4) Studies not referring to IE by carbapenem-resistant Gram-negative bacteria. Two investigators (KP, MS) used Rayyan12 to independently review the titles and abstracts of the articles that resulted from the systematic literature search and then retrieved and rescreened the full-text publications of potentially relevant articles. Any conflicts were solved with consensus. The included studies were searched for relevant articles in their references. For articles where a full-text was not available, attempts were made to communicate with the study authors to provide the full text.
Outcomes of interest
The primary outcomes of the current study were to record data on: a) the gender and age of patients with IE by carbapenem-resistant Gram-negative bacteria and b) the patients’ outcomes. Secondary outcomes were to record data on a) the infected valve, b) the clinical characteristics of the patients, c) antimicrobial resistance to other antimicrobials, and d) the treatment that was administered.
Data extraction and definitions
In general, the present study follows the standard methodology that has been used by our study group for the study of IE in different settings.13 The data were extracted from each eligible study by two investigators (MS, KP). Extracted data included the type of the study, the year the study was published, and the country where research was conducted; information on patient’s demographics (gender and age); the medical history of the patients (such as previous cardiac valve replacement or cardiac surgery, time after cardiac valve replacement); data on microbiology and the infection (such as the infected valve, information regarding pathogen identification, and presence of any complications); the definitive treatment that was administered for the infection; whether patients underwent surgery along with antimicrobials, and the outcomes (such as mortality). Data on the microbiology of infection and the association of infection with mortality was recorded according to the studies’ authors. The diagnosis of IE was also confirmed by the current study’s investigators based on the data given by each study’s authors and the modified ISCVID-Dukes’ criteria if the diagnosis of IE was at least possible (presence of at least one major and one minor criterion or presence of at least three minor criteria) or if pathology established a diagnosis of IE.14 The complications that were recorded included any clinical deterioration or organ dysfunction that was considered by each study’s authors to be associated with the IE. The quality of evidence of included studies’ outcomes was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE).15
Statistical analysis
Data are presented as number (%) for categorical variables and median (interquartile range, IQR) or mean (± standard deviation, SD) for continuous variables. Categorical data were analyzed using Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U-test for non-normally distributed variables or the t-test for normally distributed variables. The above statistics were calculated with GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Literature search
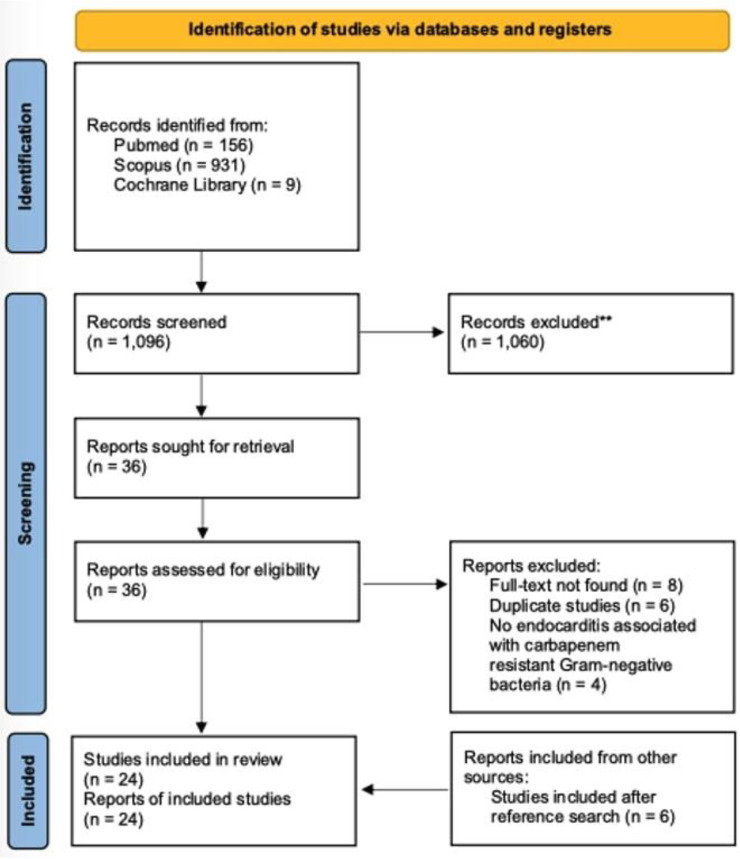
A total of 1,096 articles from PubMed, Cochrane Library, and Scopus were evaluated through the initial screening process. After reviewing the titles and abstracts, 36 articles were selected for review of the full text. From these studies, 18 were excluded from the review: eight articles could not be found, six articles were duplicates, and four were not associated with carbapenem-resistant Gram-negative bacteria. Additionally, six were included after a search of the references of the previously mentioned studies. Finally, 24 met the inclusion criteria of the present study.16-39 Figure 1 shows a graphical representation of the study inclusion procedure.
Figure 1.
Flow diagram of study inclusion
Included studies’ characteristics
The 24 studies that were eventually included in this analysis involved 26 patients. Supplementary Table 1 summarizes the characteristics of the studies included. Among them, 12 were conducted in Asia, 7 in Europe, 4 in North and South America, and 1 in Oceania. There were 21 case reports; thus, the overall quality of the evidence that contributed to this systematic review was rated as very low.15
Characteristics of IE by carbapenem-resistant Gram-negative bacteria
The age of patients with IE by carbapenem-resistant Gram-negative bacteria ranged from 18 to 83 years, the median age was 66 years, and 53.8% (14 out of 26 patients) were male. A history of a prosthetic cardiac valve was present in 38.5% (10 patients). Table 1 shows the epidemiology of patients with IE by carbapenem-resistant Gram-negative bacteria in detail.
Table 1.
Epidemiology and microbiology of infective endocarditis by carbapenem-resistant Gram-negative bacteria in total and concerning mortality
| Characteristic | All patients (n=26)* | Survived (n=16) | Died (n=10) | P value |
|---|---|---|---|---|
| Male, n (%) | 14 (53.8) | 9 (56.3) | 5 (50) | 1.000 |
| Age, median (IQR) in years | 66 (49.8-73.3) | 62 (42.3-67.8) | 67 (54-79) | 0.177 |
| Predisposing factors | ||||
| Prosthetic valve, n (%) | 10 (38.5) | 6 (37.5) | 4 (40) | 1.000 |
| CVC, n (%) | 6 (23.1) | 2 (12.5) | 4 (40) | 0.163 |
| Immunosuppression, n (%) | 5 (19.2) | 2 (12.5) | 3 (30) | 0.340 |
| Previously on antimicrobials, n (%) | 5/24 (20.8) | 2/14 (14.3) | 3 (30) | 0.615 |
| ESRD on dialysis, n (%) | 4 (15.4) | 0 (0) | 4 (40) | 0.014 |
| CIED, n (%) | 3 (11.5) | 2 (12.5) | 1 (10) | 1.000 |
| Previous IE, n (%) | 2 (7.7) | 2 (12.5) | 0 (0) | 0.508 |
| Post-cardiac surgery, n (%) | 2/24 (8.3) | 0/15 (0) | 2/9 (22.2) | 0.130 |
| Known colonization by carbapenem-resistant pathogen, n (%) | 1 (3.8) | 1 (6.3) | 0 (0) | 1.000 |
| Rheumatic fever, n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Congenital heart disease, n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Bad teeth hygiene or recent dental work, n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Microbiology | ||||
| Polymicrobial, n (%) | 1 (3.8) | 1 (6.3) | 0 (0) | 1.000 |
| Pseudomonas spp., n (%) | 10 (38.5) | 8 (50) | 2 (20) | 0.218 |
| Klebsiella spp., n (%) | 7 (26.9) | 5 (31.5) | 2 (20) | 0.668 |
| Acinetobacter spp., n (%) | 5 (19.2) | 2 (12.5) | 3 (30) | 0.340 |
| Stenotrophomonas spp., n (%) | 2 (7.7) | 1 (6.3) | 1 (10) | 1.000 |
| Achromobacter spp., n (%) | 1 (3.8) | 0 (0) | 1 (10) | 0.385 |
| Chryseobacterium spp., n (%) | 1 (3.8) | 0 (0) | 1 (10) | 0.385 |
| Antimicrobial resistance | ||||
| Quinolone, n (%) | 15/24 (62.5) | 9/14 (64.3) | 6 (60) | 1.000 |
| Aminoglycoside, n (%) | 13/24 (54.1) | 6/15 (40) | 7/9 (77.8) | 0.105 |
| Tetracyclines, n (%) | 6/15 (40) | 4/8 (50) | 2/7 (28.6) | 0.608 |
| TMP-SMX, n (%) | 4/12 (33.3) | 2/7 (28.6) | 2/5 (40) | 1.000 |
| Colistin, n (%) | 4/20 (20) | 3/13 (23.1) | 1/7 (14.3) | 1.000 |
| Chloramphenicol, n (%) | 0/9 (0) | 0/5 (0) | 0/4 (0) | 1.000 |
CIED – cardiovascular implantable electronic device; CVC – central venous catheter; ESRD – end-stage renal disease; IQR – interquartile range; NA – not applicable; TMP-SMX – trimethoprim-sulfamethoxazole.
Data are out of the number of patients stated on top unless otherwise stated.
Blood cultures were positive in all cases of IE by carbapenem-resistant Gram-negative bacteria. Infection was polymicrobial in one case (3.8%), and the concomitantly isolated pathogen was Staphylococcus epidermidis. The most commonly isolated species were Pseudomonas aeruginosa in 38.5% (10 patients), Klebsiella pneumoniae in 23.1% (6 patients), and Acinetobacter baumannii in 15.4% (4 patients). Most strains were resistant to quinolones, aminoglycosides, and tetracyclines. Detailed information on microbiology and AMR can be seen in Table 1.
Fever was the most common clinical symptom and was present in 80.8% (21 patients), while 64.7% (11 patients) had sepsis, and 20% (five out of 25 patients) had shock. Embolic phenomena occurred in 28% (7 out of 25 patients), heart failure developed in 16% (4 out of 25 patients), a paravalvular abscess occurred in 16% (4 patients), while immunological phenomena were noted in 8% (2 patients). Detailed information on diagnosis and clinical presentation of IE by carbapenem-resistant Gram-negative bacteria can be seen in Table 2.
Table 2.
Clinical characteristics, diagnosis, treatment and outcomes of patients with infective endocarditis by carbapenem-resistant Gram-negative bacteria in total and concerning mortality
| Characteristic | All patients (n=26)* | Survived (n=16) | Died (n=10) | P value |
|---|---|---|---|---|
| Method of diagnosis | ||||
| Transthoracic echocardiography, n (%) | 11/25 (44) | 8/15 (53.3) | 3 (30) | 0.414 |
| Transesophageal echocardiography, n (%) | 11/25 (44) | 5/15 (33.3) | 6 (60) | 0.241 |
| Autopsy, n (%) | 3/25 (12) | NA | 3 (30) | NA |
| Valve localization | ||||
| Aortic valve, n (%) | 15 (57.7) | 8 (50) | 7 (70) | 0.428 |
| Mitral valve, n (%) | 8 (30.8) | 5 (31.3) | 3 (30) | 1.000 |
| Tricuspid valve, n (%) | 3 (11.5) | 3 (18.8) | 0 (0) | 0.262 |
| Pulmonary valve, n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Multiple valves, n (%) | 2 (7.7) | 1 (6.3) | 1 (10) | 1.000 |
| Clinical characteristics | ||||
| Fever, n (%) | 21 (80.8) | 14 (87.5) | 7 (70) | 0.340 |
| Sepsis, n (%) | 11/17 (80.7) | 6/11 (54.5) | 5/6 (83.3) | 0.333 |
| Embolic phenomena, n (%) | 7/25 (28) | 2/15 (13.3) | 5 (50) | 0.075 |
| Shock, n (%) | 5/25 (20) | 1/15 (6.7) | 4 (40) | 0.121 |
| Paravalvular abscess, n (%) | 4/25 (16) | 1/15 (6.7) | 3 (30) | 0.267 |
| Heart failure, n (%) | 4/25 (16) | 1/15 (6.7) | 3 (30) | 0.267 |
| Immunologic phenomena, n (%) | 2/25 (8) | 1/15 (6.7) | 1 (10) | 1.000 |
| Treatment | ||||
| Duration of treatment in weeks, median (IQR) | 7.7 (6-10) | 7.7 (6-10) | NA | |
| Aminoglycoside, n (%) | 12/24 (50) | 9/15 (60) | 3/9 (33.3) | 0.400 |
| Colistin, n (%) | 10/24 (41.7) | 7/15 (46.7) | 4/9 (44.4) | 1.000 |
| Cephalosporin, n (%) | 7/24 (29.2) | 7/15 (46.7) | 0/9 (0) | 0.022 |
| Carbapenem, n (%) | 6/24 (25) | 2/15 (13.3) | 4/9 (44.4) | 0.150 |
| Quinolone, n (%) | 6/24 (25) | 2/15 (13.3) | 4/9 (44.4) | 0.150 |
| Tetracycline, n (%) | 5/24 (20.8) | 2/15 (13.3) | 3/9 (33.3) | 0.326 |
| TMP-SMX, n (%) | 4/24 (16.7) | 1/15 (6.7) | 3/9 (33.3) | 0.130 |
| Rifampicin, n (%) | 4/24 (16.7) | 2/15 (13.3) | 2/9 (22.2) | 0.615 |
| Antipseudomonal penicillin, n (%) | 3/24 (12.5) | 1/15 (6.7) | 2/9 (22.2) | 0.533 |
| Sulbactam, n (%) | 2/24 (8.3) | 1/15 (6.7) | 1/9 (11.1) | 1.000 |
| Aztreonam, n (%) | 1/24 (4.2) | 1/15 (6.7) | 0/9 (0) | 1.000 |
| Fosfomycin, n (%) | 2/24 (8.3) | 2/15 (13.3) | 0/9 (0) | 0.511 |
| Surgical management, n (%) | 14 (53.8) | 11 (68.8) | 3 (30) | 0.105 |
| Outcomes | ||||
| Deaths due to infection, n (%) | 8 (30.8) | NA | NA | |
| Deaths overall, n (%) | 10 (38.5) | NA | NA |
IQR – interquartile range; NA – not applicable; PCR – polymerase chain reaction; TMP-SMX – trimethoprim-sulfamethoxazole.
Data are out of the number of patients stated on top unless otherwise stated.
Treatment and outcomes of IE by carbapenem-resistant Gram-negative bacteria
The detailed treatment provided for the IE by carbapenem-resistant Gram-negative bacteria can be seen in Supplementary Table 1 and is also summarized in Table 2. Surgical management along with antimicrobial therapy was performed in 53.8% (14 out of 26 patients). Overall all-cause mortality was 38.5% (10 out of 26 patients) and was attributed directly to IE in 30.8% (8 patients).
Statistical analysis of IE by carbapenem-resistant Gram-negative bacteria
A statistical comparison of patients with IE by carbapenem-resistant Gram-negative who survived with those who died revealed that those who died were more likely to have a history of end-stage renal disease on dialysis and were less likely to have received treatment with cephalosporins for the episode of IE. The results of the statistical comparison can be seen in Table 1 and Table 2.
Discussion
This study described the characteristics of the patients who developed IE by carbapenem-resistant Gram-negative bacteria. The most commonly infected valve was the aortic one. The most frequent clinical findings included fever and sepsis. Aminoglycosides and colistin were the most commonly used antimicrobials, while 38.5% of patients died.
Antimicrobial resistance is emerging as a major public health issue, with significant effects on human health, due to considerable morbidity and mortality.40 The most common and important pathogens associated with AMR are the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). More specifically, carbapenems had been previously considered potent antibiotics for treating infections by Gram-negative microorganisms with AMR. However, the emergence of carbapenem resistance has significantly reduced the available options for treating these highly resistant pathogens.41-43
IE by Gram-negative bacteria is a rare condition since, in most cases, IE is caused by Gram-positive bacteria; however, in the case of IE by bacteria that do not belong to the HACEK group (Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae), mortality can be high.44-47 This is particularly important in the case of Gram-negative bacteria harboring important AMR mechanisms, as is the case of A. baumannii and Klebsiella spp.48,49 The current study is the first that addresses the issue of IE by carbapenem-resistant pathogens.
The median age of patients with IE by carbapenem-resistant Gram-negative bacteria herein was 66 years, which is relatively higher than the age at diagnosis of patients with IE by non-HACEK Gram-negative bacilli in the literature, which ranges from 40 to 63 years.45,50,51 A male predominance was noted in the present review, as was the case in patients with IE by non-HACEK Gram-negative bacilli.45,50,51 A history of a prosthetic valve was noted in 38.5% of patients with IE by carbapenem-resistant Gram-negative bacteria. That rate is close to the one reported in studies of IE by non-HACEK Gram-negative bacilli, which was within the range of 30% to 59%.45,50,51 A CIED was present in 11.5% in the current review, while, in other studies with patients suffering from IE by non-HACEK Gram-negative bacilli, the rate was as high as 29%.45,50,51 A CVC was present in 23.1% of patients with IE by carbapenem-resistant Gram-negative bacteria, a rate close to that noted in other reports with IE by non-HACEK Gram-negative bacilli, where it ranged from 17% to 20%.45,50 Recent antimicrobial use was noted in the medical history of 20.8% of patients with IE by carbapenem-resistant Gram-negative bacteria, while, in another study with data on patients suffering from IE by non-HACEK Gram-negative bacilli, the rate was 40%.51 A previous episode of IE was noted in the medical history of 7.7% in the present review. In other studies providing data on patients with IE by non-HACEK Gram-negative bacilli, that rate varied widely from 0 to 67%.45,50,51
The most commonly infected valve was the aortic one, at 57.7%, and the mitral valve, at 30.8%. These rates were different in two other reports of IE by non-HACEK Gram-negative bacilli, with the mitral valve being the most common valve infected in 31%, followed by the aortic one in 24% in the first study,45 and the aortic valve being the most commonly infected in 42%, followed by the tricuspid valve in 33% in the second report.50
As for clinical presentation, fever was the most commonly encountered symptom noted in 80.8% of patients, while 64.7% were septic. In other studies with IE by non-HACEK Gram-negative bacilli, fever was reported in 92%.45,50 Heart failure was reported in 16% of patients with IE by carbapenem-resistant Gram-negative bacteria, which is within the range of the rate seen in cases of non-HACEK Gram-negative IE which is from 8% to 37%.45,50 Embolic phenomena in IE by carbapenem-resistant Gram-negative bacteria were noted in 28%, which is close to the rate in non-HACEK Gram-negative bacilli IE which is from 17% to 65%.45,50,51 Immunological phenomena in the present review were noted in 8%, a rate lower than the one noted in IE by non-HACEK Gram-negative bacilli, which is from 25% to 27%.45,50,51 Diagnosis of a paravalvular abscess was performed in 16% of patients with IE by carbapenem-resistant Gram-negative bacteria. This was lower than the rate noted in patients suffering from IE by non-HACEK Gram-negative bacilli, which was within the range of 25% to 42%.45,50
The most frequently isolated species in the present study were Pseudomonas, Acinetobacter, and Klebsiella. This is no surprise since these three pathogens are well-known to harbor significant mechanisms of AMR, and, more specifically, many clinical isolates of all these three pathogens have been described to have carbapenem resistance.52-55 Other species were identified herein, such as Chryseobacterium or Achromobacter. However, these pathogens may be overrepresented herein since cases of IE by Pseudomonas, Acinetobacter, and Klebsiella may have been underreported relative to the more rarely isolated isolates of Chryseobacterium and Achromobacter in patients with IE.
Importantly, most carbapenem-resistant pathogens in the current study were sensitive to antimicrobials such as trimethoprim-sulfamethoxazole, tetracyclines as well as colistin. Thus, even though carbapenem may not be a viable option in infections by these pathogens, there are still some other options that could be used for treating these infections. However, not all antimicrobials may be useful for every infection. For example, tigecycline may not be an adequate option for treating IE since it may not achieve adequate levels in the blood.56 Another important consideration is that some of the included studies date back to 2000, thus, the AMR data presented in the present review may not represent the current situation. AMR rates for the abovementioned antibiotics may be even higher. For the same reason, many antimicrobials presented herein may discord with the currently published guidelines on the treatment of carbapenem-resistant pathogen infections.43 For example, some antibiotics such as cefiderocol, imipenem/cilastatin/relebactam, or meropenem/vaborbactam have been accepted for human use recently, later than the time some of the studies presented herein had been published.9,57
Based on the previously mentioned data on AMR, it is no surprise that aminoglycosides, colistin, and some beta-lactams, such as cephalosporins or carbapenems, were used for treating IE by carbapenem-resistant bacteria. The use of beta-lactams may sound controversial, given that a carbapenem-resistant pathogen would be expected to be resistant to all these antibiotics. However, most of these antibiotics were given in combination. Antimicrobial combinations are well known to have synergy in many cases of co-administration. For example, tigecycline, colistin, and ampicillin/sulbactam (due to the lack of sulbactam as a single drug in the market), or tigecycline, colistin, and meropenem are well-known combinations used for the treatment of XDR A. baumannii.10,58 On the other hand, antimicrobial combinations such as meropenem with colistin have also been extensively used in the era of carbapenem-resistant Gram-negative bacteria before the development of imipenem/cilastatin/relebactam, and meropenem/vaborbactam. However, the evidence regarding the efficacy and the safety of this antimicrobial combination is still controversial.59,60
Mortality was high, with two out of five patients dying, and most of them succumbing due to the IE by carbapenem-resistant Gram-negative bacteria. This mortality rate was higher than the one in studies including data on patients suffering from IE by non-HACEK Gram-negative bacilli, where the mortality rate was within the range of 0% to 24%.45,50,51 However, the one-year mortality in these studies was up to 30%, implying that IE by non-HACEK Gram-negative bacilli is a lethal disease.50,51 This further highlights the need to implement adequate infection control measures and antimicrobial stewardship interventions to reduce the spread of AMR and reduce the likelihood of lethal infections, including IE, by these highly resistant bacteria.61
This systematic review has some limitations that should be noted. First, it primarily includes information derived from case reports. Thus, the results presented herein should be read cautiously since the quality of evidence overall was very low. Additionally, the number of included patients is very low to derive safe conclusions. This is associated with the specific and narrow aim of this systematic review that limits the pool of included cases. Consequently, the results could have been significantly affected by publication bias. Thus, further studies are warranted to allow safe results to be drawn.
Conclusions
To conclude, this study presents the epidemiological, clinical, and microbiological characteristics of patients with IE by carbapenem-resistant Gram-negative bacteria, as well as their treatment and outcomes. Most infections were caused by Pseudomonas, Klebsiella, and Acinetobacter species. Even though AMR was high to many antibiotics, there were still some available options for treatment; however, pharmacokinetic and pharmacodynamic issues may reduce the available options for treatment. Mortality was high; thus, the development of infection control measures and antimicrobial stewardship interventions is needed to reduce the spread of AMR and the likelihood of fatal infection.
Supplementary Table 1.
Characteristics of the included studies
| Study | Number of patients | Age (years) | Gender | Site of infection n (%) | Microbiology of infection, n (%) | Treatment administered, n (%) | Infection outcomes, n (%) |
|---|---|---|---|---|---|---|---|
| Aydin et al.,16 2000 | 1 | 40 | Male | AoV | Stenotrophomonas maltophilia | Antipseudomonal penicillin TMP-SMX |
Clinical curea Deaths overall |
| Olut et al.,17 2005 | 1 | 45 | Female | AoV | Acinetobacter baumannii | Quinolone Aminoglycoside |
Clinical cure Deaths overall Deaths due to IE |
| Bomb et al.,18 2007 | 1 | 58 | Male | AoV | Chryseobacterium meningosepticum | Antipseudomonal penicillin Quinolone Rifampicin |
Clinical cure Deaths overall Deaths due to IE |
| Benenson et al.,19 2009 | 1 | 18 | Male | MV | Klebsiella pneumoniae | Colistin Aminoglycoside |
Clinical cure Deaths overall |
| Ahmadi et al.,20 2009 | 1 | 66 | Male | AoV MV |
Acinetobacter lwoffii | NR Surgical management |
Clinical cure Deaths overall |
| Katayama et al.,21 2010 | 1 | 78 | Female | MV | Stenotrophomonas maltophilia | Antipseudomonal penicillin Quinolone Tetracycline TMP-SMX Surgical management Clinical cure |
Deaths overall Deaths due to IE |
| Raymond et al.,22 2014 | 1 | 77 | Female | AoV | Klebsiella pneumoniae | Aminoglycoside Tetracycline |
Clinical cure Deaths overall Deaths due to IE |
| Naha et al.,23 2014 | 1 | 22 | Female | MV | Pseudomonas aeruginosa | Colistin Surgical management |
Clinical cure Deaths overall |
| Vergara-Lopez et al.,24 2014 | 1 | 68 | Male | AoV | Klebsiella oxytoca | Fosfomycin Aminoglycoside |
Clinical cure Deaths overall |
| Durante-Mangoni et al.,25 2014 | 3 | 55, 82, 83 | 2 male, 1 female | IED 1 (33.3) AoV 2 (66.7) |
Acinetobacter baumannii 1 (33.3) Pseudomonas aeruginosa 2 (66.7) |
Carbapenem 3 (100) Colistin 3 (100) Aminoglycoside 1 (33.3) Rifampicin 1 (33.3) TMP-SMX 1 (33.3) Surgical management 2 (66.7) |
Clinical cure 1 (33.3) Deaths overall 3 (100) Deaths due to IE 2 (66.7) |
| Chaari et al.,26 2015 | 1 | 67 | Male | AoV | Klebsiella pneumoniae | Colistin | Clinical cure Deaths overall |
| Patel et al.,27 2015 | 1 | 51 | Male | MV | Acinetobacter baumannii | NA | Clinical cure Deaths overall Deaths due to IE |
| Chen et al.,28 2015 | 1 | 56 | Female | TrV | Acinetobacter baumannii | Sulbactam Cephalosporin Surgical management |
Clinical cure Deaths overall |
| Domitrovic et al.,29 2016 | 1 | 58 | Female | AoV | Pseudomonas aeruginosa | Carbapenem Colistin Aminoglycoside Rifampicin Tetracycline Surgical management |
Clinical cure Deaths overall |
| Kantarcioglu et al.,30 2016 | 1 | 50 | Female | TrV | Klebsiella pneumoniae | Tetracycline Surgical management |
Clinical cure Deaths overall |
| Xia et al.,31 2018 | 1 | 66 | Female | AoV MV |
Achromobacter xylosoxidans | Quinolone TMP-SMX |
Clinical cure Deaths overall Deaths due to IE |
| Gürtler et al.,32 2019 | 1 | 66 | Male | AoV | Pseudomonas aeruginosa | Cephalosporin Quinolone Aminoglycoside Surgical management |
Clinical cure Deaths overall |
| Prescott et al.,33 2019 | 1 | 66 | Male | MV | Pseudomonas aeruginosa | Cephalosporin Colistin Fosfomycin Surgical management |
Clinical cure Deaths overall |
| Peghin et al.,34 2019 | 1 | 49 | Male | IED | Pseudomonas aeruginosa | Cephalosporin Aminoglycoside Surgical management |
Clinical cure Deaths overall |
| Edgeworth et al.,35 2019 | 1 | 78 | Female | AoV | Pseudomonas aeruginosa | Cephalosporin Aminoglycoside Carbapenem Colistin Surgical management |
Clinical cure Deaths overall |
| Dvoretsky et al.,36 2021 | 1 | 68 | Male | AoV | Klebsiella pneumoniae | Sulbactam Carbapenem Aminoglycoside Tetracycline |
Clinical cure Deaths overall Deaths due to IE |
| Alghoribi et al.,37 2021 | 1 | 40 | Female | TrV | Klebsiella pneumoniae | Cephalosporin Aztreonam |
Clinical cure Deaths overall |
| Lima et al.,38 2022 | 1 | 72 | Male | AoV | Pseudomonas aeruginosa | Cephalosporin Aminoglycoside Surgical management |
Clinical cure Deaths overall |
| Walczak et al.,39 2023 | 1 | 80 | Female | MV | Pseudomonas aeruginosa | Colistin Quinolone Aminoglycoside Rifampicin Surgical management |
Clinical cure Deaths overall |
Defined as clinical resolution of the infection as a result of treatment.
AoV – aortic valve; IE – infective endocarditis; IED – implantable electronic device; MV – mitral valve; NA – not applicable; TMP-SMX – trimethoprim-sulfamethoxazole; TrV – tricuspid valve.
Footnotes
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–86. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320:72–83. doi: 10.1001/jama.2018.7596. [DOI] [PubMed] [Google Scholar]
- 3.Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222–32. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- 4.Shah ASV, McAllister DA, Gallacher P, et al. Incidence, microbiology, and outcomes in patients hospitalized with infective endocarditis. Circulation. 2020;141:2067–77. doi: 10.1161/CIRCULATIONAHA.119.044913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cresti A, Chiavarelli M, Scalese M, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7:27–35. doi: 10.21037/cdt.2016.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papakonstantinou PE, Samonis G, Andrianaki AM, et al. Epidemiology, microbiological and clinical features, treatment, and outcomes of infective endocarditis in Crete, Greece. Infect Chemother. 2018;50:21–8. doi: 10.3947/ic.2018.50.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R, Fang X, Zhang J, et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: a systematic review and meta-analysis. BMJ Open. 2021;11:e054971. doi: 10.1136/bmjopen-2021-054971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69:S565–75. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofteridis DP, Andrianaki AM, Maraki S, et al. Treatment pattern, prognostic factors, and outcome in patients with infection due to pan-drug-resistant gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2020;39:965–70. doi: 10.1007/s10096-019-03784-9. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannou P, Kourtidis M, Mytilinis DO, Psyllaki A, Baliou S, Kofteridis D. Whipple's disease-associated infective endocarditis: a systematic review. Infect Dis (Lond) 2023;55:447–57. doi: 10.1080/23744235.2023.2214610. [DOI] [PubMed] [Google Scholar]
- 14.Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-ISCVID criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis. 2023;77:518–26. doi: 10.1093/cid/ciad271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydin K, Köksal I, Kaygusuz S, Kaklikkaya I, Caylan R, Ozdemir R. Endocarditis caused by Stenotrophomonas maltophilia. Scand J Infect Dis. 2000;32:427–30. doi: 10.1080/003655400750045060. [DOI] [PubMed] [Google Scholar]
- 17.Olut AI, Erkek E. Early prosthetic valve endocarditis due to Acinetobacter baumannii: a case report and brief review of the literature. Scand J Infect Dis. 2005;37:919–21. doi: 10.1080/00365540500262567. [DOI] [PubMed] [Google Scholar]
- 18.Bomb K, Arora A, Trehan N. Endocarditis due to Chryseobacterium meningosepticum. Indian J Med Microbiol. 2007;25:161–2. doi: 10.1016/S0255-0857(21)02180-0. [DOI] [PubMed] [Google Scholar]
- 19.Benenson S, Navon-Venezia S, Carmeli Y, et al. Carbapenem-resistant Klebsiella pneumoniae endocarditis in a young adult. Successful treatment with gentamicin and colistin. Int J Infect Dis. 2009;13:e295–298. doi: 10.1016/j.ijid.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi H, Boroumand MA, Anvari MS, Karimi A, Moshtaghi N. Left-sided endocarditis associated with multi-drug resistance Acinetobacter lwoffii. J Tehran Heart Cent. 2009;4:189–92. [Google Scholar]
- 21.Katayama T, Tsuruya Y, Ishikawa S. Stenotrophomonas maltophilia endocarditis of prosthetic mitral valve. Intern Med. 2010;49:1775–7. doi: 10.2169/internalmedicine.49.3701. [DOI] [PubMed] [Google Scholar]
- 22.Raymond T, Wiesen J, Rehm S, Auron M. Carbapenem-resistant Klebsiella pneumoniae prosthetic valve endocarditis: a feared combination of technology and emerging pathogens. Infect Dis Clin Pract. 2014;22:113–5. doi: 10.1097/IPC.0b013e318287c881. [DOI] [Google Scholar]
- 23.Naha S, Naha K, Acharya V, Hande HM, Vivek G. Community-acquired multidrug-resistant Gram-negative bacterial infective endocarditis. BMJ Case Rep. 2014;2014:bcr2014204176. doi: 10.1136/bcr-2014-204176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergara-López S, Domínguez MC, Conejo MC, Pascual Á, Rodríguez-Baño J. Prolonged treatment with large doses of fosfomycin plus vancomycin and amikacin in a case of bacteraemia due to methicillin-resistant Staphylococcus epidermidis and IMP-8 metallo-β-lactamase-producing Klebsiella oxytoca. J Antimicrob Chemother. 2015;70:313–5. doi: 10.1093/jac/dku341. [DOI] [PubMed] [Google Scholar]
- 25.Durante-Mangoni E, Andini R, Agrusta F, et al. Infective endocarditis due to multidrug resistant gram-negative bacilli: single centre experience over 5 years. Eur J Intern Med. 2014;25:657–61. doi: 10.1016/j.ejim.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Chaari A, Mnif B, Chtara K, et al. Efficacy of tigecycline-colistin combination in the treatment of carbapenem-resistant Klebsiella pneumoniae endocarditis. J Glob Antimicrob Resist. 2015;3:214–6. doi: 10.1016/j.jgar.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Patel G, Perez F, Hujer AM, et al. Fulminant endocarditis and disseminated infection caused by carbapenem-resistant Acinetobacter baumannii in a renal-pancreas transplant recipient. Transpl Infect Dis. 2015;17:289–96. doi: 10.1111/tid.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Cao H, Lu H, Qiu Z, He J. Bioprosthetic tricuspid valve endocarditis caused by Acinetobacter baumannii complex, a case report and brief review of the literature. J Cardiothorac Surg. 2015;10:149. doi: 10.1186/s13019-015-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domitrovic TN, Hujer AM, Perez F, et al. Multidrug resistant Pseudomonas aeruginosa causing prosthetic valve endocarditis: a genetic-based chronicle of evolving antibiotic resistance. Open Forum Infect Dis. 2016;3((4)):ofw188. doi: 10.1093/ofid/ofw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantarcioglu B, Bekoz HS, Olgun FE, et al. Allogeneic stem cell transplantation in a blast-phase chronic myeloid leukemia patient with carbapenem-resistant Klebsiella pneumoniae tricuspid valve endocarditis: a case report. Mol Clin Oncol. 2016;5:347–50. doi: 10.3892/mco.2016.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia R, Otto C, Zeng J, et al. Achromobacter endocarditis in native cardiac valves - an autopsy case report and review of the literature. Cardiovasc Pathol. 2018;36:6–10. doi: 10.1016/j.carpath.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Gürtler N, Osthoff M, Rueter F, et al. Prosthetic valve endocarditis caused by Pseudomonas aeruginosa with variable antibacterial resistance profiles: a diagnostic challenge. BMC Infect Dis. 2019;19:530. doi: 10.1186/s12879-019-4164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prescott A, Kennedy S, Howard P, et al. Ceftolozane-tazobactam in combination with fosfomycin for treatment of MDR/XDR P. aeruginosa infective endocarditis. Clin Infect Pract. 2019;2:100011. doi: 10.1016/j.clinpr.2019.100011. [DOI] [Google Scholar]
- 34.Peghin M, Maiani M, Castaldo N, et al. Ceftolozane/tazobactam for the treatment of MDR Pseudomonas aeruginosa left ventricular assist device infection as a bridge to heart transplant. Infection. 2018;46:263–5. doi: 10.1007/s15010-017-1086-0. [DOI] [PubMed] [Google Scholar]
- 35.Edgeworth JD, Merante D, Patel S, et al. Compassionate use of cefiderocol as adjunctive treatment of native aortic valve endocarditis due to extremely drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2019;68:1932–4. doi: 10.1093/cid/ciy963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvoretsky LI, Yakovlev SV, Suvorova MP, Varyasin VV, Stepanchenko AP, Karnaushkina MA. A rare case of pure white cell aplasia in a patient with thymoma complicated by infective endocarditis. Arch Balk Med Union. 2021;56:257–62. doi: 10.31688/ABMU.2021.56.2.17. [DOI] [Google Scholar]
- 37.Alghoribi MF, Alqurashi M, Okdah L, et al. Successful treatment of infective endocarditis due to pandrug-resistant Klebsiella pneumoniae with ceftazidime-avibactam and aztreonam. Sci Rep. 2021;11:9684. doi: 10.1038/s41598-021-89255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima O, Sousa A, Filgueira A, et al. Successful ceftazidime-avibactam therapy in a patient with multidrug-resistant Pseudomonas aeruginosa infective endocarditis. Infection. 2022;50:1039–41. doi: 10.1007/s15010-022-01834-7. [DOI] [PubMed] [Google Scholar]
- 39.Walczak A, McCarthy K, Paterson DL. A contemporary case series of Pseudomonas aeruginosa infective endocarditis. Medicine (Baltimore) 2023;102:e32662. doi: 10.1097/MD.0000000000032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulingam T, Parumasivam T, Gazzali AM, et al. Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170:106103. doi: 10.1016/j.ejps.2021.106103. [DOI] [PubMed] [Google Scholar]
- 41.Peri AM, Doi Y, Potoski BA, Harris PNA, Paterson DL, Righi E. Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis. 2019;94:413–25. doi: 10.1016/j.diagmicrobio.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Jean SS, Harnod D, Hsueh PR. Global threat of carbapenem-resistant Gram-negative bacteria. Front Cell Infect Microbiol. 2022;12:823684. doi: 10.3389/fcimb.2022.823684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28:521–47. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Bouza E, Muñoz P, Burillo A. Gram-negative endocarditis: disease presentation, diagnosis and treatment. Curr Opin Infect Dis. 2021;34:672–80. doi: 10.1097/QCO.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 45.Morpeth S, Murdoch D, Cabell CH, et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829–35. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 46.Ertugrul Mercan M, Arslan F, Ozyavuz Alp S, et al. Non-HACEK Gram-negative bacillus endocarditis. Med Mal Infect. 2019;49:616–20. doi: 10.1016/j.medmal.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Sebillotte M, Boutoille D, Declerck C, et al. Non-HACEK gram-negative bacilli endocarditis: a multicentre retrospective case-control study. Infect Dis (Lond) 2023;55:599–606. doi: 10.1080/23744235.2023.2226212. [DOI] [PubMed] [Google Scholar]
- 48.Ioannou P, Mavrikaki V, Kofteridis DP. Infective endocarditis by Acinetobacter species: a systematic review. J Chemother. 2021;33:203–15. doi: 10.1080/1120009X.2020.1812804. [DOI] [PubMed] [Google Scholar]
- 49.Ioannou P, Miliara E, Baliou S, Kofteridis DP. Infective endocarditis by Klebsiella species: a systematic review. J Chemother. 2021;33:365–74. doi: 10.1080/1120009X.2021.1888025. [DOI] [PubMed] [Google Scholar]
- 50.Loubet P, Lescure FX, Lepage L, et al. Endocarditis due to gram-negative bacilli at a French teaching hospital over a 6-year period: clinical characteristics and outcome. Infect Dis (Lond) 2015;47:889–95. doi: 10.3109/23744235.2015.1075660. [DOI] [PubMed] [Google Scholar]
- 51.Veve MP, McCurry ED, Cooksey GE, Shorman MA. Epidemiology and outcomes of non-HACEK infective endocarditis in the southeast United States. PLoS One. 2020;15:e0230199. doi: 10.1371/journal.pone.0230199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32:609–16. doi: 10.1097/QCO.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 53.Katchanov J, Asar L, Klupp EM, et al. Carbapenem-resistant Gram-negative pathogens in a German university medical center: prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations. PLoS One. 2018;13:e0195757. doi: 10.1371/journal.pone.0195757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008-2010) Diagn Microbiol Infect Dis. 2012;73:354–60. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents. 2014;43:328–34. doi: 10.1016/j.ijantimicag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58:1221–9. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- 57.Zhanel GG, Lawrence CK, Adam H, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 58.Karakonstantis S, Ioannou P, Samonis G, Kofteridis DP. Systematic review of antimicrobial combination options for pandrug-resistant Acinetobacter baumannii. Antibiotics (Basel) 2021;10:1344. doi: 10.3390/antibiotics10111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaye KS, Marchaim D, Thamlikitkul V, et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evid. 2023;2:10. doi: 10.1056/EVIDoa2200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katip W, Uitrakul S, Oberdorfer P. A comparison of colistin versus colistin plus meropenem for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients: a propensity score-matched analysis. Antibiotics. 2020;9:647. doi: 10.3390/antibiotics9100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López-Viñau T, Peñalva G, García-Martínez L, et al. Impact of an antimicrobial stewardship program on the incidence of carbapenem resistant Gram-negative bacilli: an interrupted time-series analysis. Antibiotics (Basel) 2021;10:586. doi: 10.3390/antibiotics10050586. [DOI] [PMC free article] [PubMed] [Google Scholar]