Abstract
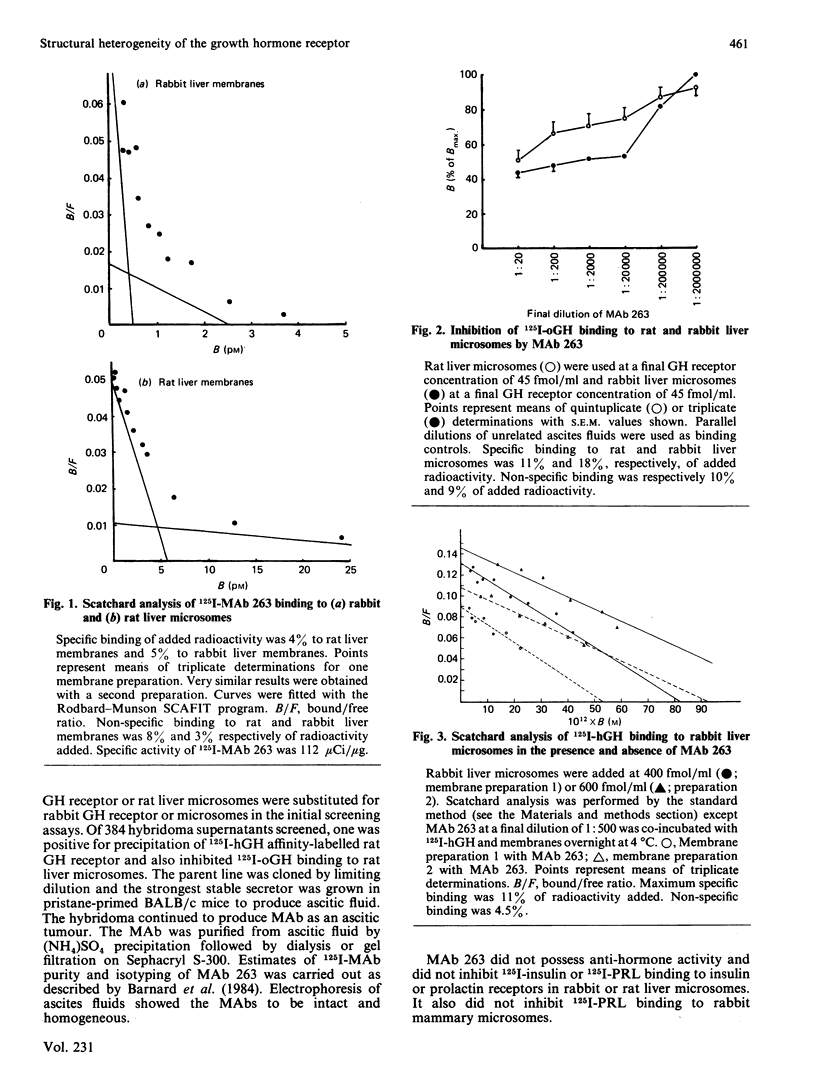
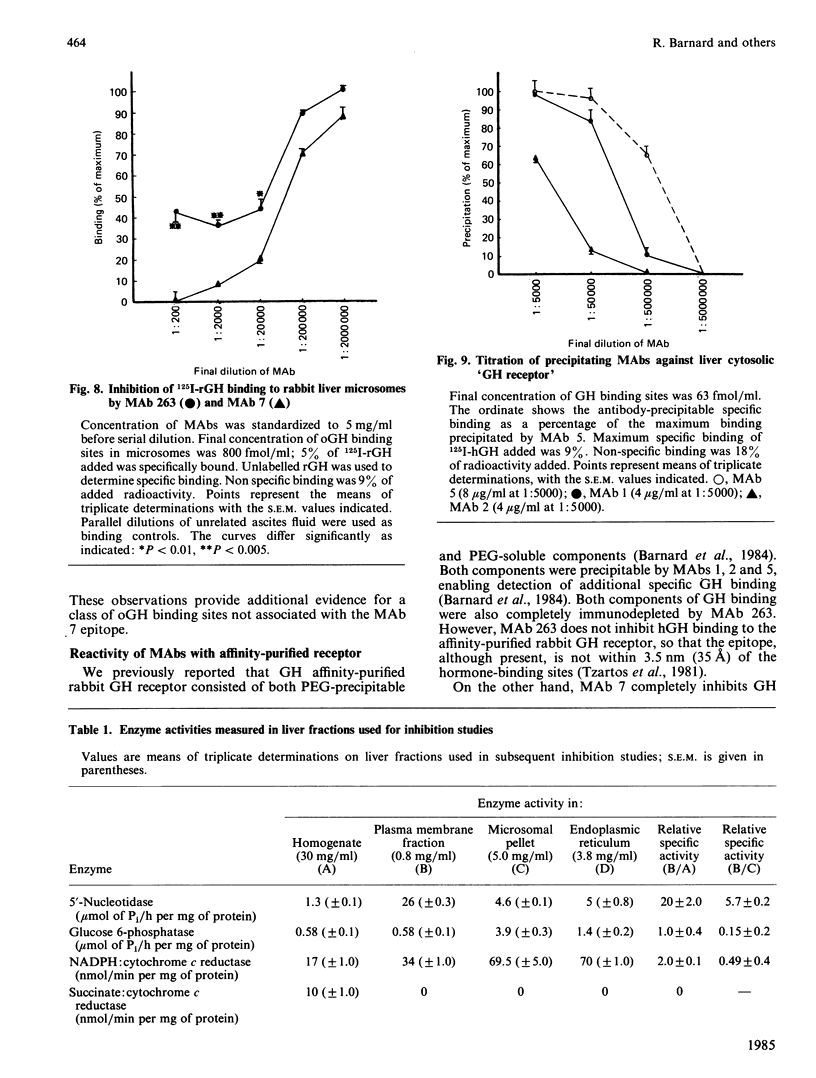
We describe the use of four monoclonal antibodies (MAbs) to the rabbit liver growth hormone (GH) receptor and one raised against purified rat liver GH receptor to characterize liver receptor subtypes which differ in their hormone-binding regions. The anti-(rat liver GH receptor) MAb both inhibited and precipitated rat and rabbit GH receptors, but only one-half of 125I-oGH (ovine GH) binding to liver microsomes could be inhibited by excess antibody. Conversely, only one-half of 125I-anti-(rat GH receptor) MAb binding was inhibited by excess oGH and Scatchard plots for this MAb exhibited two components. Although only 50% of 125I-oGH binding to membranes was inhibited by this MAb, all solubilized receptor could be immunoprecipitated. We postulate two epitopes for the anti-(rat GH receptor) MAb, one located at the hormone-binding site (inhibitory site) and one elsewhere (immunoprecipitating site). A second, rabbit-specific antibody (MAb 7) inhibited 85% of hormone binding but only 30% of 125I-anti-(rat GH receptor) MAb binding to rabbit liver microsomes. A combination of this MAb with the anti-(rat GH receptor) MAb totally inhibited 125I-oGH binding. MAb 7 alone totally inhibited 125I-rat GH binding to rabbit liver microsomes, as it did with 125I-oGH binding to purified receptor. On the basis of these results and others we postulate three types of GH receptor in rabbit liver membranes and ascribe approximate extents of 125I-oGH binding to each. A cytosolic 'GH receptor' which is not poly(ethylene glycol)-precipitable is shown to share five epitopes with 'type 2' microsomal receptors. Purified plasma membrane and endoplasmic reticulum fractions derived from a rabbit liver microsomal preparation have identical antigenic characteristics with respect to the GH-binding region, indicating that the heterogeneity we describe is not related to receptor processing. Of the three types of GH receptor in the plasma membrane of the rabbit (and possibly rat) we postulate that one (type 1) corresponds to the GH receptor involved in stimulating growth and possesses all of the epitopes studied here. A second (type 2) appears to be identical with the cytosolic 'GH receptor' and lacks the epitope for the anti-(rat GH receptor) MAb in the hormone binding site region. A third (type 3) does not possess the epitope for the inhibitory anti-(rabbit GH receptor) MAb, appears not to bind rat GH and is lost during purification. The availability of type-specific MAbs will facilitate assignment of specific functions to liver receptor subtypes which mediate the multiple functions of GH.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit T., Barkey R. J., Gavish M., Youdim M. B. Induction of prolactin (PRL) receptors by PRL in the rat lung and liver. Demonstration and characterization of a soluble receptor. Endocrinology. 1984 Feb;114(2):545–552. doi: 10.1210/endo-114-2-545. [DOI] [PubMed] [Google Scholar]
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984 Nov;115(5):1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- Baumann G., Abramson E. C. Urinary growth hormone in man: evidence for multiple molecular forms. J Clin Endocrinol Metab. 1983 Feb;56(2):305–311. doi: 10.1210/jcem-56-2-305. [DOI] [PubMed] [Google Scholar]
- Chawla R. K., Parks J. S., Rudman D. Structural variants of human growth hormone: biochemical, genetic, and clinical aspects. Annu Rev Med. 1983;34:519–547. doi: 10.1146/annurev.me.34.020183.002511. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Frigeri L. G., Peterson S. M., Lewis U. J. The 20,000-dalton structural variant of human growth hormone: lack of some early insulin-like effects. Biochem Biophys Res Commun. 1979 Dec 14;91(3):778–782. doi: 10.1016/0006-291x(79)91947-8. [DOI] [PubMed] [Google Scholar]
- Grichting G., Levy L. K., Goodman H. M. Relationship between binding and biological effects of human growth hormone in rat adipocytes. Endocrinology. 1983 Sep;113(3):1111–1120. doi: 10.1210/endo-113-3-1111. [DOI] [PubMed] [Google Scholar]
- Hart I. C., Blake L. A., Chadwick P. M., Payne G. A., Simmonds A. D. The heterogeneity of bovine growth hormone. Extraction from the pituitary of components with different biological and immunological properties. Biochem J. 1984 Mar 1;218(2):573–581. doi: 10.1042/bj2180573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. P., Tokuhiro E., Simpson J. S., Friesen H. G. 20K is bound with high affinity by one rat and one of two rabbit growth hormone receptors. Endocrinology. 1983 Nov;113(5):1904–1906. doi: 10.1210/endo-113-5-1904. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Conkling M. A., Goodman H. M. Human growth hormone: a multigene family. Cell. 1982 Jun;29(2):285–286. doi: 10.1016/0092-8674(82)90144-1. [DOI] [PubMed] [Google Scholar]
- Paladini A. C., Peña C., Poskus E. Molecular biology of growth hormone. CRC Crit Rev Biochem. 1983;15(1):25–56. doi: 10.3109/10409238309102800. [DOI] [PubMed] [Google Scholar]
- Pass M. A., Pugh M. W., Findlay L. Studies on the mechanism of toxicity of reduced lantadene A in rats. Biochem Pharmacol. 1981 Jun 15;30(12):1433–1437. doi: 10.1016/0006-2952(81)90363-4. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Vodian M. A., Hughes J. P., Nicoll C. S. Electrophoretic separation of forms of rat growth hormone with different bioassay and radioimmunoassay activities: comparison of intraglandular and secreted forms. Life Sci. 1978 Dec 11;23(24):2373–2382. doi: 10.1016/0024-3205(78)90295-3. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Simpson J. S., Hughes J. P., Friesen H. G. A monoclonal antibody to the growth hormone receptor of rabbit liver membranes. Endocrinology. 1983 Jun;112(6):2137–2141. doi: 10.1210/endo-112-6-2137. [DOI] [PubMed] [Google Scholar]
- Stowell C. P., Kuhlenschmidt T. B., Hoppe C. A. A fluorescamine assay for submicrogram quantities of protein in the presence of Triton X-100. Anal Biochem. 1978 Apr;85(2):572–580. doi: 10.1016/0003-2697(78)90256-7. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Waters M. J., Friesen H. G. Purification and partial characterization of a nonprimate growth hormone receptor. J Biol Chem. 1979 Jul 25;254(14):6815–6825. [PubMed] [Google Scholar]
- Waters M. J., Lusins S., Friesen H. G. Immunological and physicochemical evidence for tissue specific prolactin receptors in the rabbit. Endocrinology. 1984 Jul;115(1):1–10. doi: 10.1210/endo-115-1-1. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S. I., Stevenson J. L., Herington A. C. Identification of a rabbit liver cytosolic binding protein for human growth hormone. Biochem J. 1984 Aug 1;221(3):617–622. doi: 10.1042/bj2210617. [DOI] [PMC free article] [PubMed] [Google Scholar]


