Abstract
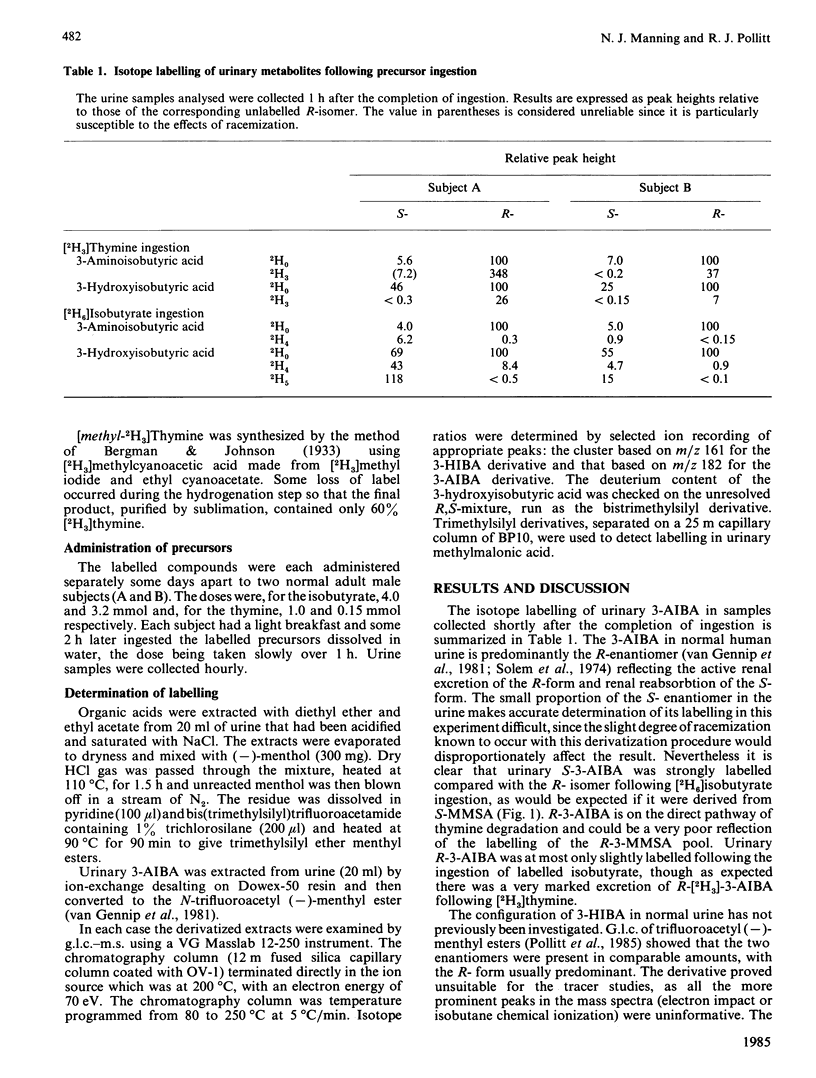
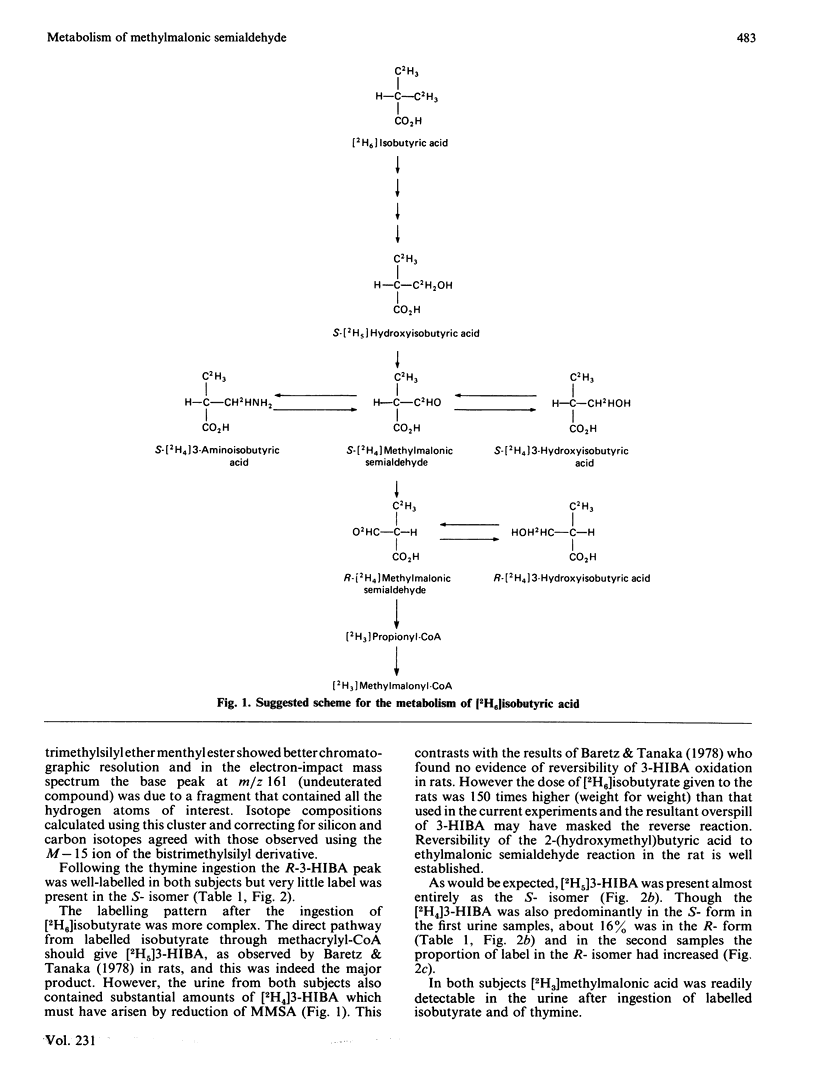
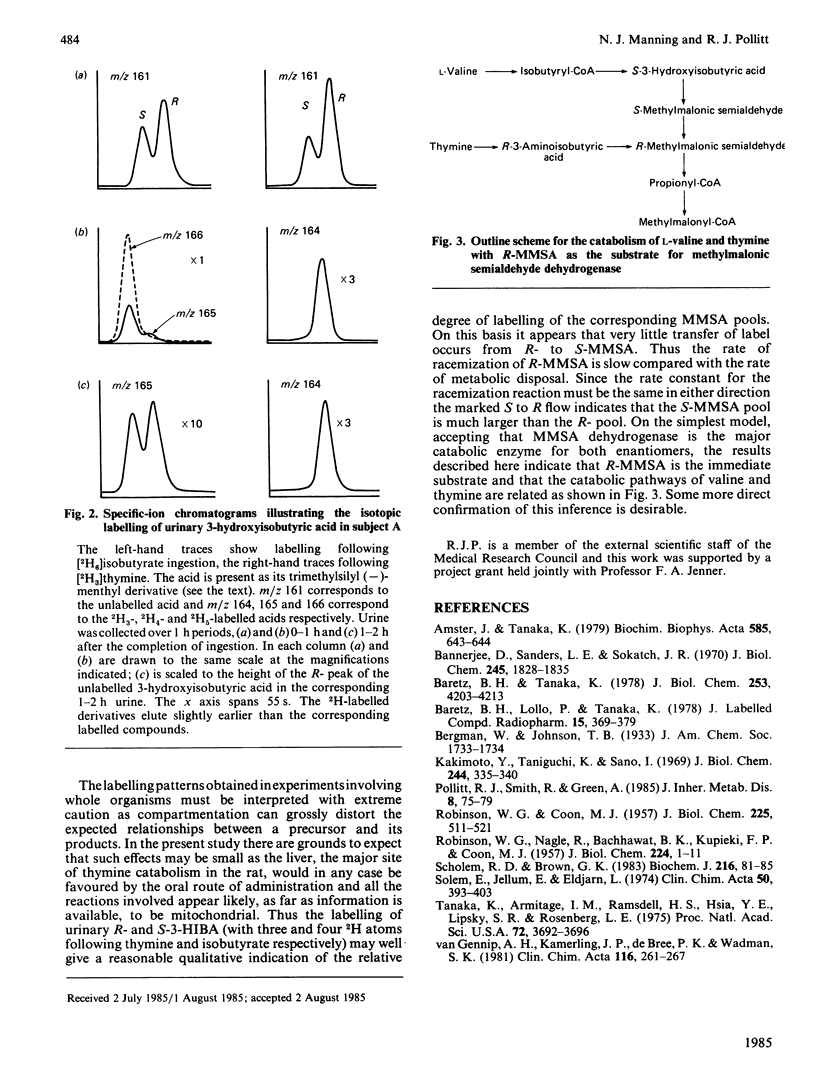
Two human subjects were given separate oral doses of sodium [2H6]isobutyrate and [methyl-2H3]thymine and the labelling patterns of urinary metabolites were determined. Ingestion of deuterated isobutyrate resulted in the excretion of 2H5-labelled S-3-hydroxyisobutyric acid, formed on the direct catabolic pathway, and of S- and R-[2H4]-3-hydroxyisobutyric acids, formed by the reduction of S- and R-methylmalonic semialdehydes respectively. Only the R-enantiomer of urinary 3-hydroxyisobutyric acid was labelled by thymine. This labelling pattern indicates a flow from S- to R-methylmalonic semialdehyde, suggesting that the R-enantiomer is the substrate of methylmalonic semialdehyde dehydrogenase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster J., Tanaka K. Isolation and identification of S(+)-3-hydroxyisobutyric acid in the urine of rats loaded with isobutyric acid. Biochim Biophys Acta. 1979 Jul 18;585(4):643–644. doi: 10.1016/0304-4165(79)90196-x. [DOI] [PubMed] [Google Scholar]
- BACHHAWAT B. K., COON M. J., KUPIECKI F. P., NAGLE R., ROBINSON W. G. Coenzyme A thiol esters of isobutyric, methacrylic, and beta-hydroxyisobutyric acids as intermediates in the enzymatic degradation of valine. J Biol Chem. 1957 Jan;224(1):1–11. [PubMed] [Google Scholar]
- Bannerjee D., Sanders L. E., Sokatch J. R. Properties of purified methylmalonate semialdehyde dehydrogenase of Pseudomonas aeruginosa. J Biol Chem. 1970 Apr 10;245(7):1828–1835. [PubMed] [Google Scholar]
- Baretz B. H., Tanaka K. Metabolism in rats in vivo of isobutyrates labeled with stable isotopes at various positions. Identification of propionate as an obligate intermediate. J Biol Chem. 1978 Jun 25;253(12):4203–4213. [PubMed] [Google Scholar]
- Kakimoto Y., Taniguchi K., Sano I. D-beta-aminoisobutyrate:pyruvate aminotransferase in mammalian liver and excretion of beta-aminoisobutyrate by man. J Biol Chem. 1969 Jan 25;244(2):335–340. [PubMed] [Google Scholar]
- Pollitt R. J., Green A., Smith R. Excessive excretion of beta-alanine and of 3-hydroxypropionic, R- and S-3-aminoisobutyric, R- and S-3-hydroxyisobutyric and S-2-(hydroxymethyl)butyric acids probably due to a defect in the metabolism of the corresponding malonic semialdehydes. J Inherit Metab Dis. 1985;8(2):75–79. doi: 10.1007/BF01801669. [DOI] [PubMed] [Google Scholar]
- ROBINSON W. G., COON M. J. The purification and properties of beta-hydroxyisobutyric dehydrogenase. J Biol Chem. 1957 Mar;225(1):511–521. [PubMed] [Google Scholar]
- Scholem R. D., Brown G. K. Metabolism of malonic semialdehyde in man. Biochem J. 1983 Oct 15;216(1):81–85. doi: 10.1042/bj2160081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Armitage I. M., Ramsdell H. S., Hsia Y. E., Lipsky S. R., Rosenberg L. E. [13C]Valine metabolism in methylmalonicacidemia using nuclear magnetic resonance: propinonate as an obligate intermediate. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3692–3696. doi: 10.1073/pnas.72.9.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gennip A. H., Kamerling J. P., de Bree P. K., Wadman S. K. Linear relationship between the R- and S-enantiomers of a beta-aminoisobutyric acid in human urine. Clin Chim Acta. 1981 Nov 11;116(3):261–267. doi: 10.1016/0009-8981(81)90045-0. [DOI] [PubMed] [Google Scholar]


