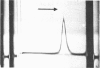
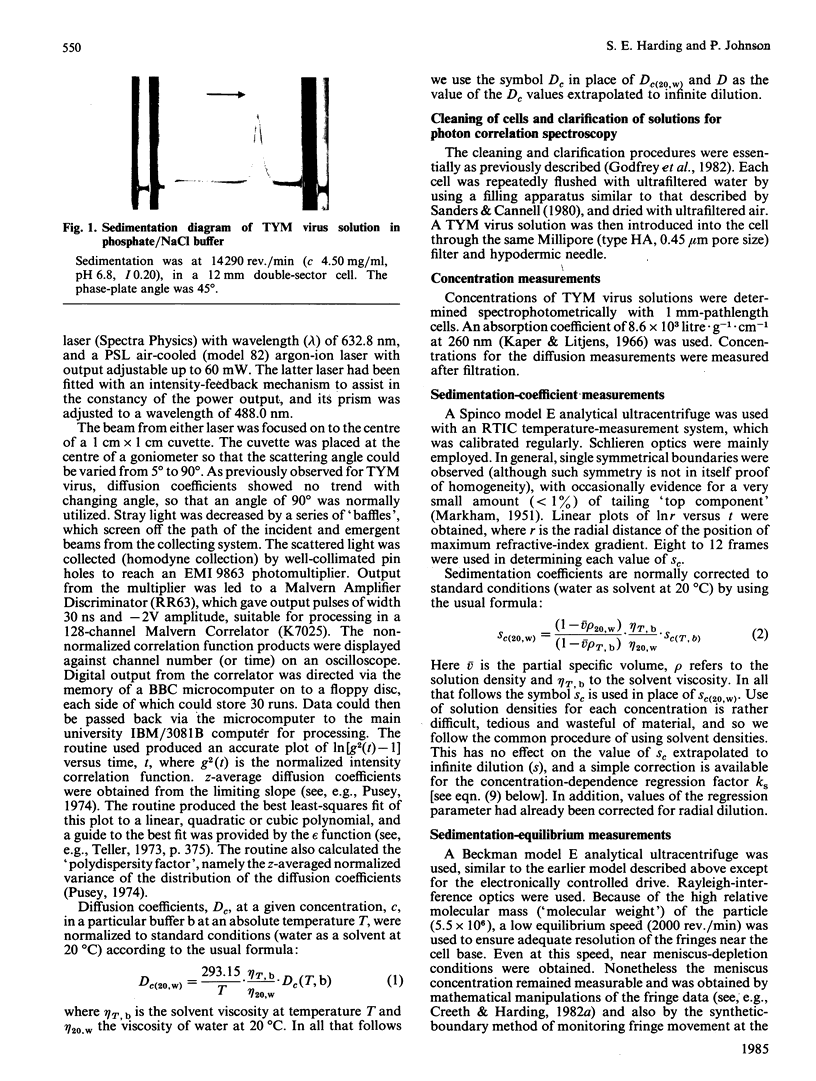
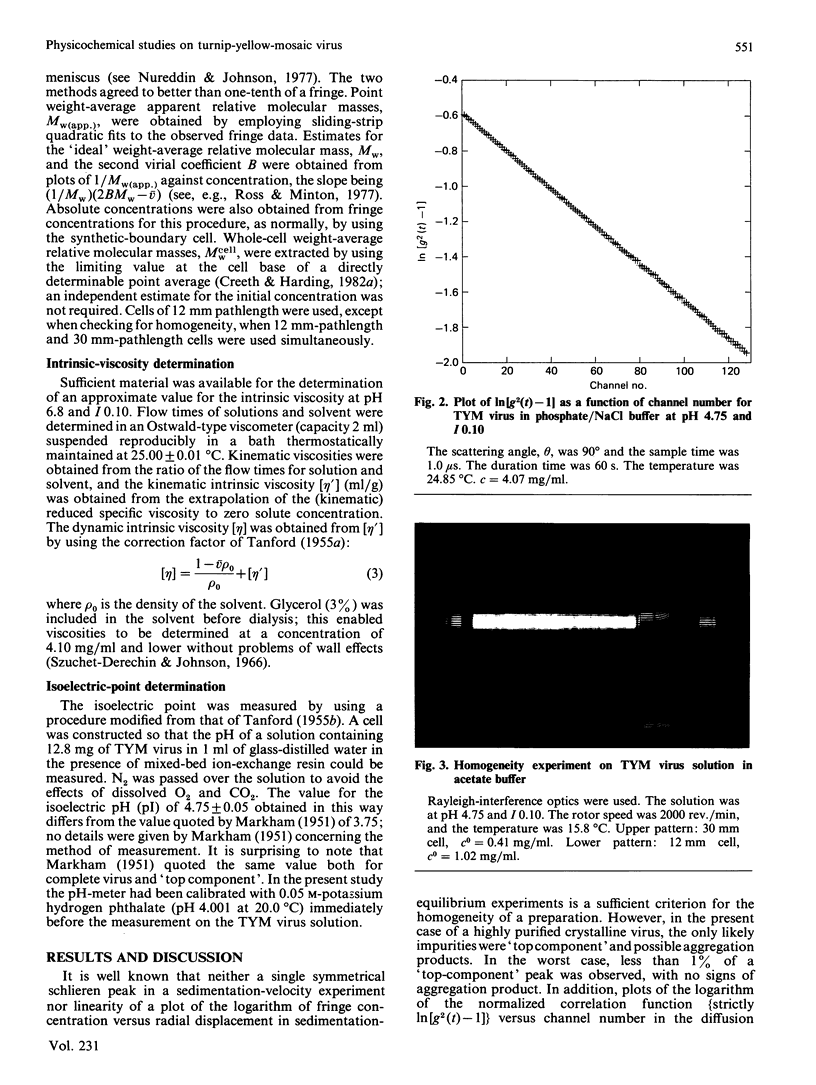
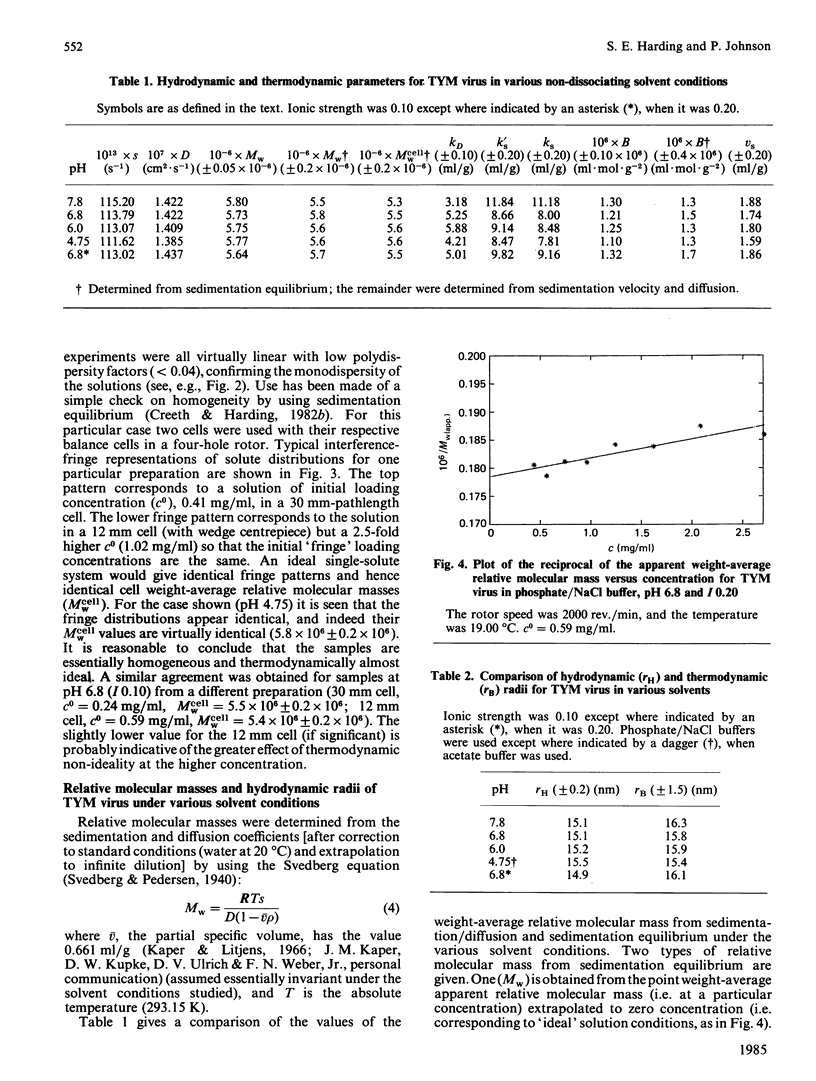
Abstract
Turnip-yellow-mosaic virus, with its stable, highly spherical and monodisperse character, was chosen as a suitable model substance with which to test hydrodynamic theories of transport. Sedimentation coefficients, diffusion coefficients (obtained through photon correlation spectroscopy) and viscosities were measured accurately as a function of concentration in well-defined and nearly neutral buffer systems. Ancillary information was also obtained from very-low-speed sedimentation-equilibrium experiments. The coefficients expressing the variation in sedimentation and diffusion coefficients with weight concentration were obtained, and by combination with other data it was possible to avoid assumptions concerning solvation and transform such regression coefficients into the form appropriate to volume fractions. Some measure of support for Batchelor's [(1972) J. Fluid Mech. 52, 245-268] calculations was thus obtained, but over most of the pH range the coefficients were significantly smaller than those calculated from his theory. It seems likely that electrostatic interactions are responsible for the discrepancies. Hydrodynamic radii (from diffusion coefficients) were in very fair agreement with those calculated from the thermodynamic excluded-volume term, but were higher than indicated by electron microscopy and X-ray diffraction, a discrepancy ascribable to solvation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creeth J. M., Harding S. E. A simple test for macromolecular heterogeneity in the analytical ultracentrifuge. Biochem J. 1982 Sep 1;205(3):639–641. doi: 10.1042/bj2050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Harding S. E. Some observations on a new type of point average molecular weight. J Biochem Biophys Methods. 1982 Dec;7(1):25–34. doi: 10.1016/0165-022x(82)90033-1. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Knight C. G. On the estimation of the shape of macromolecules from sedimentation and viscosity measurements. Biochim Biophys Acta. 1965 Jul 22;102(2):549–558. doi: 10.1016/0926-6585(65)90145-7. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Arrangement of protein subunits and the distribution of nucleic acid in turnip yellow mosaic virus. II. Electron microscopic studies. J Mol Biol. 1966 Jan;15(1):344–364. doi: 10.1016/s0022-2836(66)80231-0. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Johnson P. Quasi-elastic light scattering studies on dormant and germinating Bacillus subtilis spores. Biochem J. 1984 May 15;220(1):117–123. doi: 10.1042/bj2200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
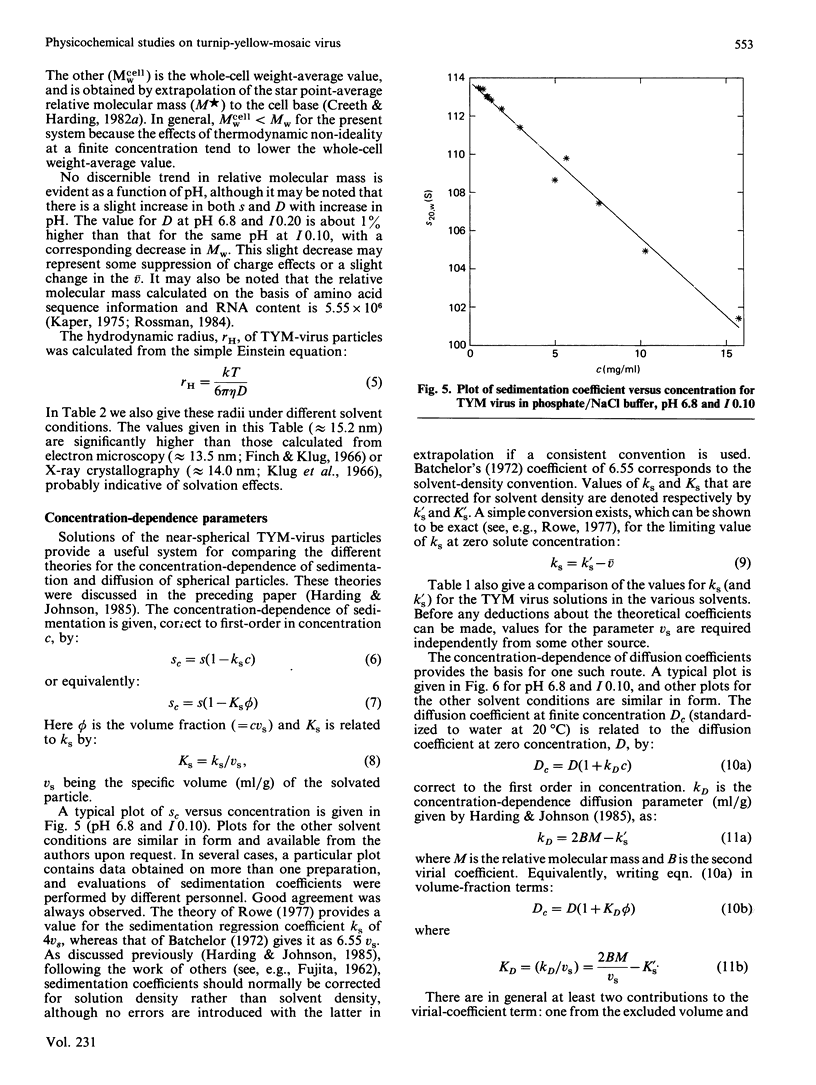
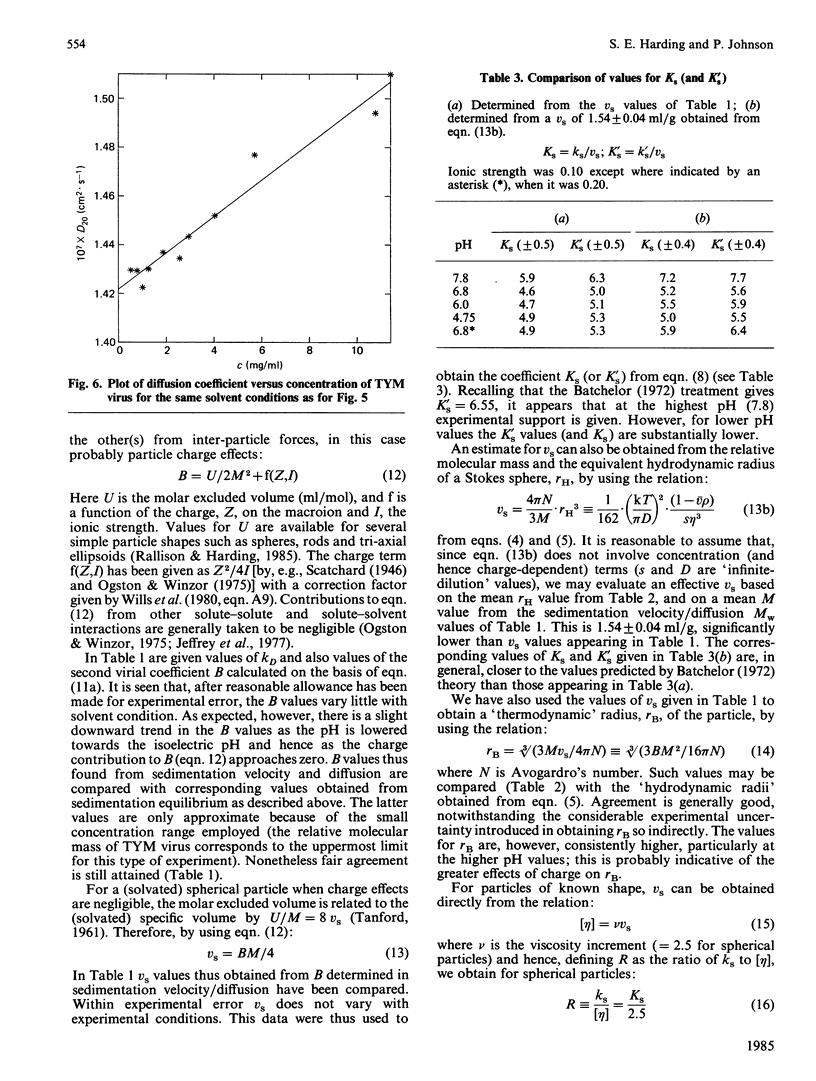
- Harding S. E., Johnson P. The concentration-dependence of macromolecular parameters. Biochem J. 1985 Nov 1;231(3):543–547. doi: 10.1042/bj2310543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPER J. M. Preparation and characterization of artificial top component from turnip yellow mosaic virus. J Mol Biol. 1960 Dec;2:425–433. doi: 10.1016/s0022-2836(60)80052-6. [DOI] [PubMed] [Google Scholar]
- KAPER J. M. Protein components from turnip yellow mosaic virus. Nature. 1960 Apr 16;186:219–220. doi: 10.1038/186219a0. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Litjens E. C. The ribonucleic acid content of turnip yellow mosaic virus. Biochemistry. 1966 May;5(5):1612–1617. doi: 10.1021/bi00869a023. [DOI] [PubMed] [Google Scholar]
- Klug A., Longley W., Leberman R. Arrangement of protein subunits and the distribution of nucleic acid in turnip yellow mosaic virus. I. X-ray diffraction studies. J Mol Biol. 1966 Jan;15(1):315–343. doi: 10.1016/s0022-2836(66)80230-9. [DOI] [PubMed] [Google Scholar]
- Nureddin A., Johnson P. Ultracentrifugal studies of human luteinizing hormone and its subunits: dependence on protein concentration and ionic strength. Biochemistry. 1977 Apr 19;16(8):1730–1737. doi: 10.1021/bi00627a033. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Analysis of non-ideal behavior in concentrated hemoglobin solutions. J Mol Biol. 1977 May 25;112(3):437–452. doi: 10.1016/s0022-2836(77)80191-5. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The evolution and function of biological molecular assemblies: from catalase to viruses. Ninth Keilin Memorial lecture. Biochem Soc Trans. 1984 Apr;12(2):115–126. doi: 10.1042/bst0120115. [DOI] [PubMed] [Google Scholar]
- Teller D. C. Characterization of proteins by sedimentation equilibrium in the analytical ultracentrifuge. Methods Enzymol. 1973;27:346–441. doi: 10.1016/s0076-6879(73)27017-9. [DOI] [PubMed] [Google Scholar]
- Wills P. R., Nichol L. W., Siezen R. J. The indefinite self-association of lysozyme: consideration of composition-dependent activity coefficients. Biophys Chem. 1980 Feb;11(1):71–82. doi: 10.1016/0301-4622(80)85009-5. [DOI] [PubMed] [Google Scholar]