Abstract
Plasmid clones containing cDNA coding for the B-chain of human Clq were isolated from a liver cDNA library. The longest cDNA insert isolated contained all the coding sequence for amino acid residues B1 to B226 plus a 3' non-translated region of 264 nucleotides that extended into the poly(A) tail, thus accounting for 950 nucleotides of the mRNA. The B-chain mRNA was estimated by Northern-blot analysis to be 1.46 kb (kilobases) long, which indicated that approx. 500 bases were not accounted for in the cDNA clone. A cosmid clone containing the C1q-B chain gene was isolated from a human genomic DNA library. The precise 5' limit of gene was not established, but from the data available it appears that the gene is approx. 2.6 kb long. The coding sequence for residues B1 to B226 in the gene is interrupted by one intron, of 1.1 kb, which is located within the codon coding for glycine at position B36. This glycine residue is located in the middle of the triple-helical regions found in C1q at exactly the position where there is an unusual structural feature, i.e. a bend in each of the helical regions brought about by the interruption of the Gly-Xaa-Yaa repeating triplet sequences in the A- and C-chains and the presence of an 'extra' triplet in the B-chain. Nucleotide sequencing of the 5' end of the gene indicates the presence of a predominantly hydrophobic stretch of 29 amino acids, immediately before residue B1, which could serve as a signal peptide.
Full text
PDF


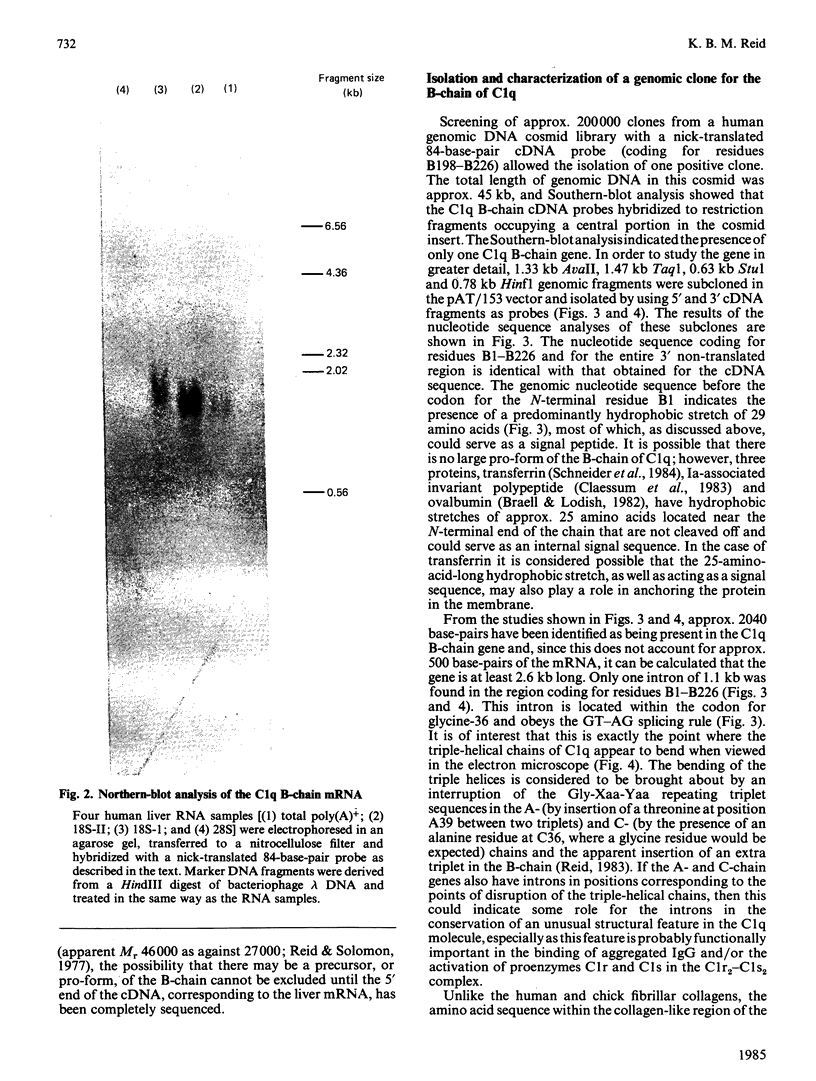
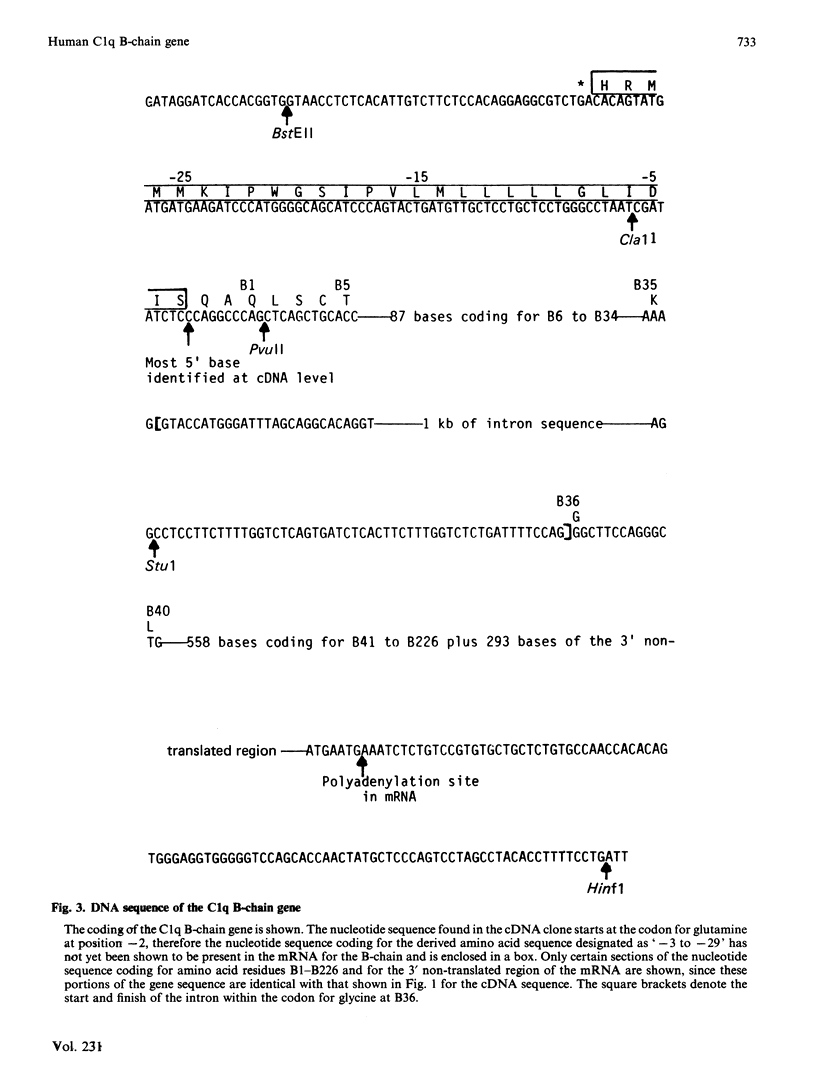
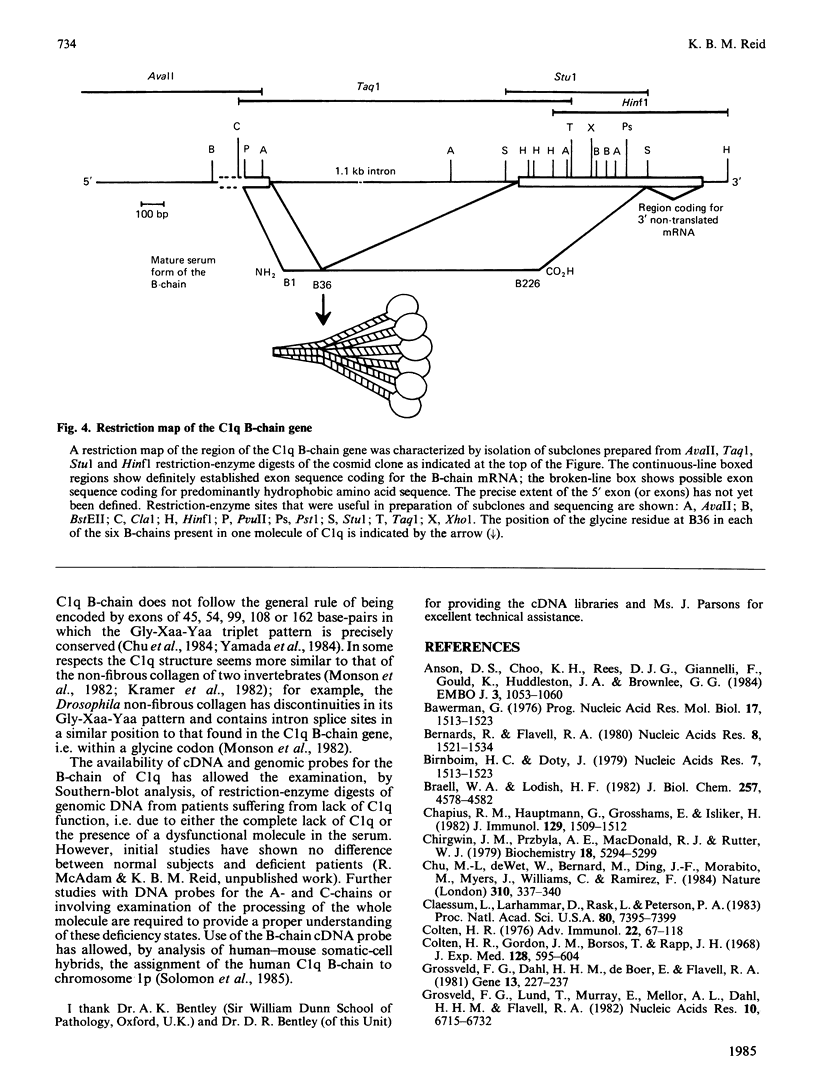

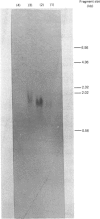
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Ovalbumin utilizes an NH2-terminal signal sequence. J Biol Chem. 1982 Apr 25;257(8):4578–4582. [PubMed] [Google Scholar]
- Chapuis R. M., Hauptmann G., Grosshans E., Isliker H. Structural and functional studies in C1q deficiency. J Immunol. 1982 Oct;129(4):1509–1512. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ding J. F., Morabito M., Myers J., Williams C., Ramirez F. Human pro alpha 1(I) collagen gene structure reveals evolutionary conservation of a pattern of introns and exons. 1984 Jul 26-Aug 1Nature. 310(5975):337–340. doi: 10.1038/310337a0. [DOI] [PubMed] [Google Scholar]
- Claesson L., Larhammar D., Rask L., Peterson P. A. cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7395–7399. doi: 10.1073/pnas.80.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H. R. Biosynthesis of complement. Adv Immunol. 1976;22:67–118. doi: 10.1016/s0065-2776(08)60548-9. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Gordon J. M., Borsos T., Rapp H. J. Synthesis of the first component of human complement in vitro. J Exp Med. 1968 Oct 1;128(4):595–604. doi: 10.1084/jem.128.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Loos M. Biosynthesis of the collagen-like C1q molecule and its receptor functions for Fc and polyanionic molecules on macrophages. Curr Top Microbiol Immunol. 1983;102:1–56. doi: 10.1007/978-3-642-68906-2_1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Natzle J., Friedman J., McCarthy B. J. Expression and novel structure of a collagen gene in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1761–1765. doi: 10.1073/pnas.79.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. M., Colten H. R., Bing D. H. The first component of complement. A quantitative comparison of its biosynthesis in culture by human epithelial and mesenchymal cells. J Exp Med. 1978 Oct 1;148(4):1007–1019. doi: 10.1084/jem.148.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Bentley D. R., Wood K. J. Cloning and characterization of the complementary DNA for the B chain of normal human serum C1q. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):345–354. doi: 10.1098/rstb.1984.0095. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J., Frampton J. Completion of the amino acid sequences of the A and B chains of subcomponent C1q of the first component of human complement. Biochem J. 1982 Jun 1;203(3):559–569. doi: 10.1042/bj2030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Proteins involved in the activation and control of the two pathways of human complement. Biochem Soc Trans. 1983 Jan;11(1):1–12. doi: 10.1042/bst0110001. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Solomon E. Biosynthesis of the first component of complement by human fibroblasts. Biochem J. 1977 Dec 1;167(3):647–660. doi: 10.1042/bj1670647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Thompson E. O. Amino acid sequence of the N-terminal 108 amino acid residues of the B chain of subcomponent C1q of the first component of human complement. Biochem J. 1978 Sep 1;173(3):863–868. doi: 10.1042/bj1730863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Thompson R. A. Characterization of a non-functional form of C1q found in patients with a genetically linked deficiency of C1q activity. Mol Immunol. 1983 Oct;20(10):1117–1125. doi: 10.1016/0161-5890(83)90121-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Thompson R. A., Haeney M., Reid K. B., Davies J. G., White R. H., Cameron A. H. A genetic defect of the C1q subcomponent of complement associated with childhood (immune complex) nephritis. N Engl J Med. 1980 Jul 3;303(1):22–24. doi: 10.1056/NEJM198007033030107. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Liau G., Mudryj M., Obici S., de Crombrugghe B. Conservation of the sizes for one but not another class of exons in two chick collagen genes. 1984 Jul 26-Aug 1Nature. 310(5975):333–337. doi: 10.1038/310333a0. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Kitajima H., Tanabe S., Ochi T., Shinkai H. Effect of age on C1q and C3 levels in human serum and their presence in colostrum. Immunology. 1978 Sep;35(3):523–530. [PMC free article] [PubMed] [Google Scholar]