Abstract
Amphipods in the parvorder Lysianassidira are scavengers, often collected in sediment, coral rubble, algae, or among other invertebrates. Members of the parvorder have a head that is deeper than long, large coxae, lacinia mobilis present only on the left molar, and a mitten-shaped gnathopod 2 propodus with a long ischium. Nine species from two families within the parvorder are documented from Bocas del Toro, Panama. This research documents range extensions for eight species and an identification key to the species of Caribbean Lysianassidira of Panama is provided.
Key words: Bocas del Toro, identification key, Lysianassidae, Lysianassoidea, Tryphosidae
Resumen Abstract
Los anfípodos del parvorden Lysianassidira son carroñeros, a menudo recolectados en sedimentos, escombros de coral, algas o entre otros invertebrados. Los miembros del parvorden tienen una cabeza que es más profunda que larga, con coxas grandes, lacinia mobilis presenta solo en el molar izquierdo y un gnatópodo 2 en forma de manopla con un isquion largo. Nueve especies de dos familias dentro del parvorder están documentadas en Bocas del Toro, Panamá. Esta investigación documenta extensiones de rango para ocho especies y se proporciona una clave de identificación para las especies de Lysianassidira caribeña de Panamá.
Introduction
Parvorder Lysianassidira Dana, 1849 is comprised of 1243 species around the world, with several listed as incertae sedis (Horton et al. 2024). Members of the parvorder are characterized by having the head that is deeper than long, antenna 1 with callynophore, large coxae, lacinia mobilis present only on the left molar, and a distally mitten-shaped gnathopod 2 propodus with a long ischium (Lowry and Myers 2017). The parvorder contains 33 families of amphipods: Alicellidae Lowry & DeBroyer, 2008 (17 spp.), Parargissidae Lowry & Myers, 2017 (two spp.), Podoprionidae Lowry & Stoddart, 1996 (four spp.), Valettidae Stebbing, 1888 (two spp.), Valettiopsidae Lowry & DeBroyer, 2008 (12 spp.), Vemanidae Lowry & Myers, 2017 (five spp.), Stegocephalidae Dana, 1852 (110 spp.), Adeliellidae Lowry & Myers, 2017 (three spp.), Amaryllididae Lowry & Stoddart, 2002 (37 spp.), Cebocaridae Lowry & Stoddart, 2011 (15 spp.), Cyclocaridae Lowry & Stoddart, 2011 (four spp.), Cyphocarididae Lowry & Stoddart, 1997 (21 spp.), Eurytheneidae Stoddart & Lowry, 2004 (10 spp.), Hirondelleidae Lowry & Stoddart, 2010a (20 spp.), Lysianassidae Dana, 1849 (130 spp.), Opisidae Lowry & Stoddart, 1995 (19 spp.), Scopelocheiridae Lowry & Stoddart, 1997 (27 spp.), Tryphosidae Lowry & Stoddart, 1997 (389 spp.), Uristidae Hurley, 1963 (190 spp.), Acidostomatidae Stoddart & Lowry, 2012 (11 spp.), Ambasiidae Lowry & Myers, 2017 (three spp.), Aristiidae Lowry & Stoddart, 1997 (42 spp.), Conicostomatidae Lowry & Stoddart, 2012 (19 spp.), Derjugianidae Lowry & Myers, 2017 (one sp.), Endevouridae Lowry & Stoddart, 1997 (19 spp.), Izinkalidae Lowry & Stoddart, 2010c (two spp.), Kergueleniidae Lowry & Stoddart, 2010d (26 spp.), Lepidepecreellidae Stoddart & Lowry, 2010 (12 spp.), Pakynidae Lowry & Myers, 2017 (38 spp.), Sophrosynidae Lowry & Stoddart, 2010b (14 spp.), Thoriellidae Lowry & Stoddart, 2011 (seven spp.), Trischizostomatidae Lilljeborg, 1865 (18 spp.), Wandinidae Lowry & Stoddart, 1990 (four spp.). Only 30 species in the parvorder have been previously reported from the Caribbean Sea, representing ten families (Aristiidae, Cyphocarididae, Endevouridae, Eurythenidae, Lysianassidae, Parargissidae, Stegocephalidae, Tryphosidae, Uristidae, Vemanidae). Four species, Concarnesconcavus (Shoemaker, 1933), Eclecticuseclecticus Lowry & Stoddart, 1997, Paracentromedoncarabicus Barnard, 1964, and Vemanacompressa Barnard, 1964 have been previously reported from Caribbean Panama (LeCroy et al. 2009; Miloslavich et al. 2010; Martín et al. 2013). Miloslavich et al. (2010) listed Parargissagalatheaamericana Barnard, 1961 from Caribbean Panama without locality details, but Barnard (1961) stated that it was collected from the Pacific. Andres (1977) documented P.galatheaamericana from the eastern Atlantic, but the author can find no reports of this species from the Caribbean and, thus, do not include it herein.
Within the parvorder Lysianassidira, nine species of amphipods were collected from Bocas del Toro, Panama, with representatives from the families Lysianassidae and Tryphosidae. Regional diagnoses for each species collected during this study are provided herein. An identification key is provided to distinguish between the Lysianassidira species known from the Caribbean waters of Panama.
Methods
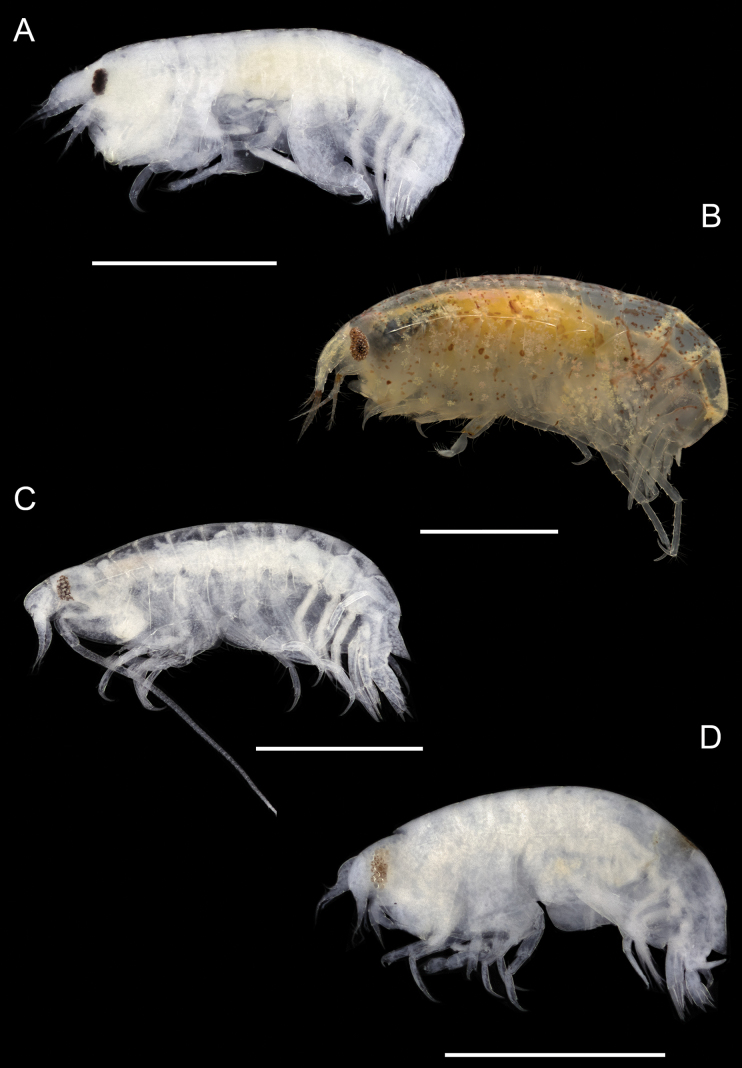
Coral rubble, sand, algae, and sponges were collected by hand and placed into buckets or plastic bags from various sites around Bocas del Toro, Panama at depths of 0–15 m. Coral rubble, sand, and algae were elutriated with freshwater to remove amphipods, and sponges were sorted through by hand. Live amphipods were sorted to morphospecies, placed in clove oil for imaging, and preserved in 99.5% EtOH for later examination. Preserved specimens were transferred to glycerol, measured from the tip of the rostrum to the base of the telson, and dissected under a stereomicroscope. Specimens were illustrated using a Meiji MT5900L phase contrast microscope with an Olympus U-DA drawing tube. Illustrations were digitally inked following Coleman (2003) in Adobe Illustrator 2024 using a Wacom® Intuos Pro Pen Tablet. Abbreviations used in figures are as follows: Hd, head; Mx2, maxilla 2; G, gnathopod; P, pereopod; E, epimeron; Ur: urosome; U, uropod; T, telson. Size ranges of each species collected from Bocas del Toro, Panama are provided at the beginning of each material examined section. Specimens are deposited in the Smithsonian Institution, U.S. National Museum of Natural History (USNM) and the Gulf Coast Research Laboratory Museum (GCRL).
Results
Parvorder Lysianassidira Dana, 1849
Superfamily Lysianassoidea Dana, 1849
Family Lysianassidae Dana, 1849
Genus. Aruga
Holmes, 1908
9D0EAF1E-B7F0-5A65-A3A7-13BE75CC28BD
Diagnosis.
Antenna 1 with strong callynophore in male and female. Antenna 2 flagellum elongate in male. Epistome not produced; upper lip produced. Maxilla 2 inner plate narrow. Gnathopod 1 simple. Gnathopod 2 minutely chelate. Uropod 2 inner ramus with dorsal notch, gradually narrowing distally. Uropod 3 outer ramus 2-articulate. Telson entire.
. Aruga holmesi
J.L. Barnard, 1955
6C8AAD1B-8190-566E-88C5-A683B59D4695
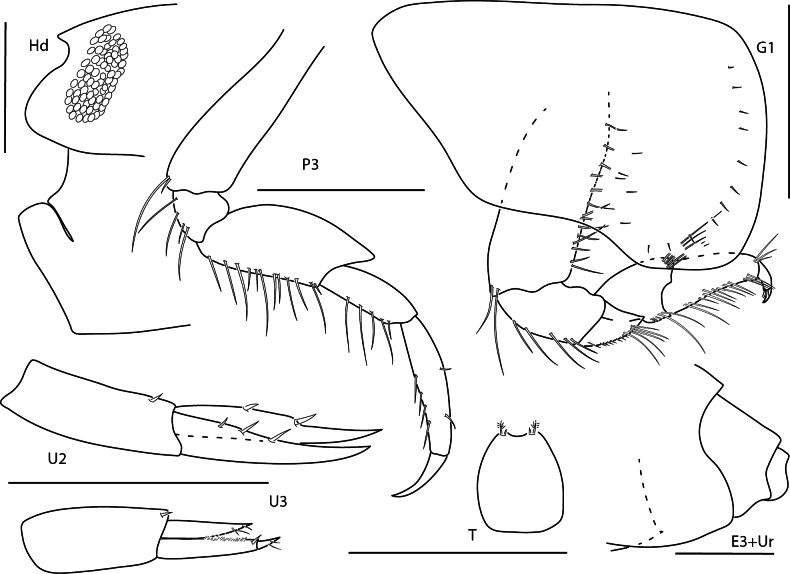
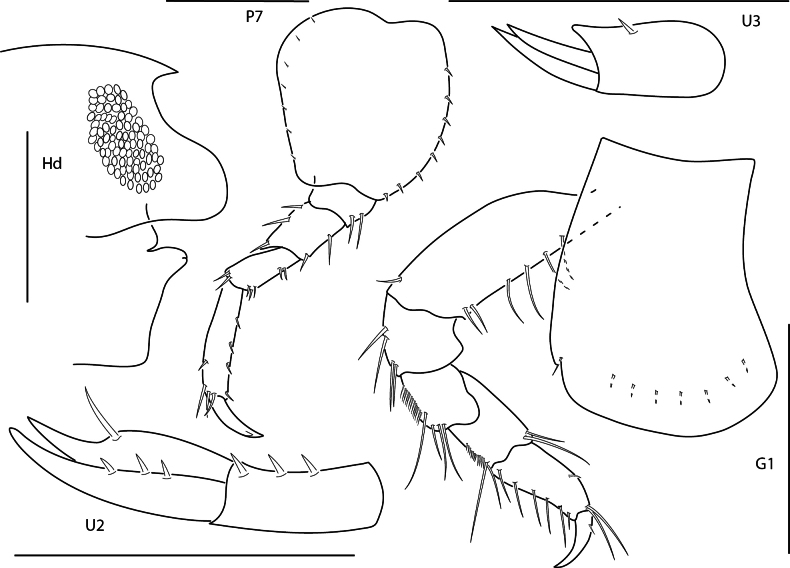
Figure 1.
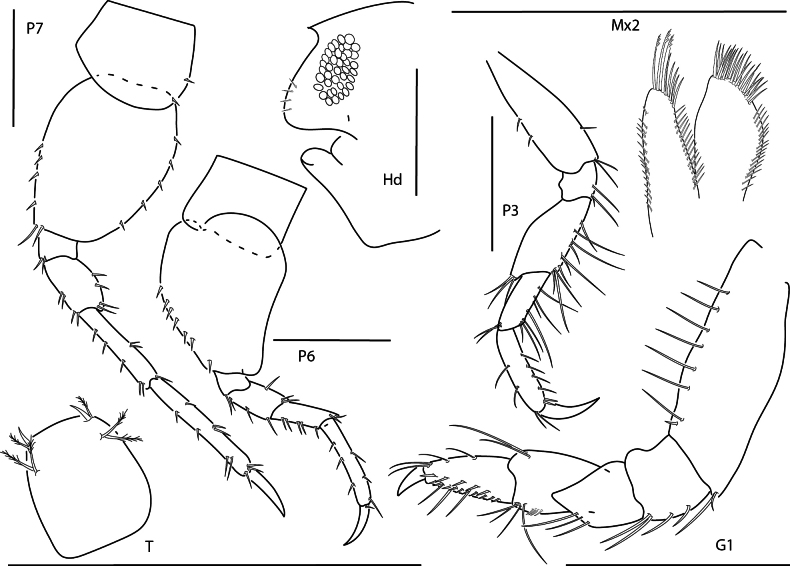
Arugaholmesi, female, 4.8 mm, head, epistome and upper lip, pereopod 3, gnathopod 1 lateral, uropod 2, uropod 3, telson, epimeron 3 and urosome. Scale bars: 0.5 mm.
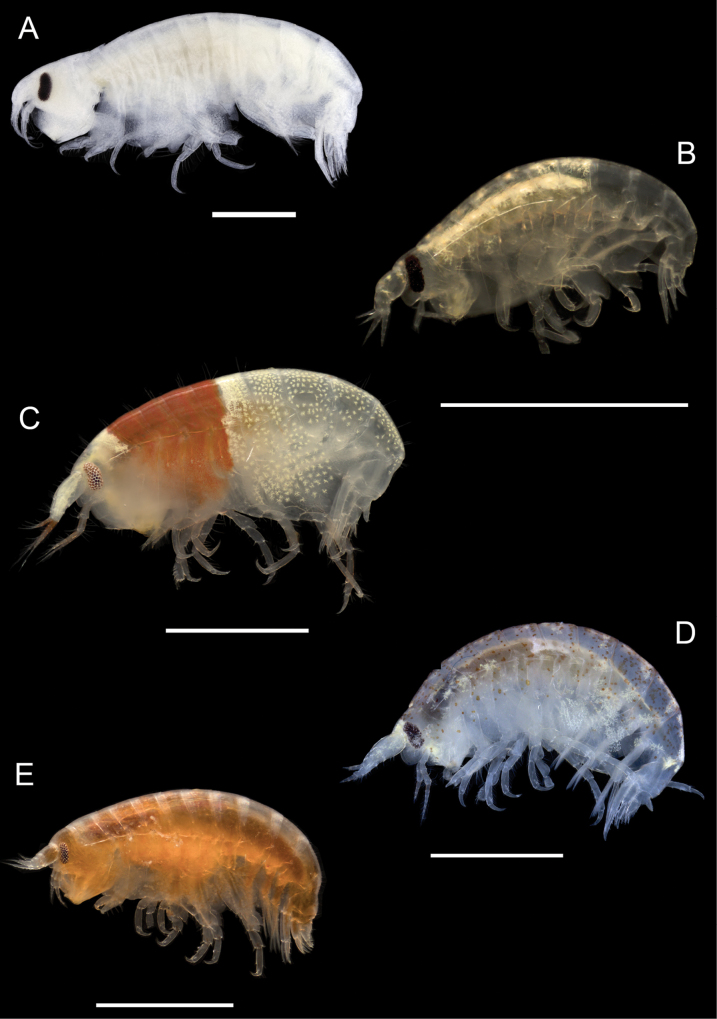
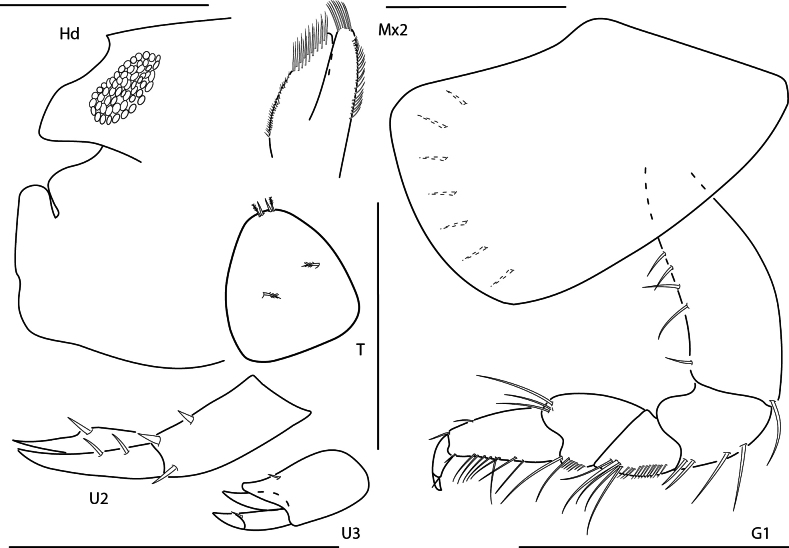
Figure 10.
Photographs of live specimens unless noted AArugaholmesi (ethanol preserved specimen) BBonassabonairensisCConcarnesconcavusDLysianopsishummelinckiELysianopsisozona. Scale bars: 1.0 mm.
Aruga holmesi J.L. Barnard, 1955: 100, pls 27, 28; J.L. Barnard 1958: 90; J.L. Barnard 1959: 18; Gurjanova 1962: 299–301, figs 98, 99; J.L. Barnard 1964: 79, chart 1; Barnard and Karaman 1991: 469; Lowry and Stoddart 1997: 47–53, figs 17–20; LeCroy 2007: 575, fig. 497.
Lysianopsis holmesi : Hurley 1963: 74, 75, fig. 21b.
Lysianassa holmesi : J.L. Barnard 1966a: 25; J.L. Barnard 1966b: 69; J.L. Barnard 1979: 12, 130; Austin 1985: 600; Stepien and Brusca 1985: 97–101, fig. 2F; Stretch 1985: 129–133.
Material examined.
Panama • 4.8 mm • 1 ♀; Bocas del Toro, Crawl Cay; 9.2376°N, 82.1438°W; depth 1.5–3 m, among coral rubble; 11 Aug 2021; K.N. White leg.; USNM 1739772.
Diagnosis.
Upper lip projecting well beyond epistome; epistome concave. Gnathopod 1 propodus posterodistal margin slightly concave. Epimeron 3 posteroventral corner subquadrate, without tooth. Uropod 3 peduncle length at least 2 × width. Telson distal margin truncate, slightly emarginate, with two short setae on each side.
Distribution.
USA: Folly Island, South Carolina; Florida from Perdido Key to the lower Florida Keys (LeCroy 2007); Pacific California (Lowry and Stoddart 1997); Ecuador (Lowry and Stoddart 1997); Panama: Pacific side of Isthmus of Panama (Lowry and Stoddart 1997); Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with coral rubble and seagrass beds at depths of 1.5–120 m. Panamanian specimens agree closely with previous descriptions of the species. Lowry and Stoddart (1997) recorded this species from the Gulf of Mexico for the first time, noting that it was previously only known from the Pacific side of the Isthmus of Panama. Panamanian specimens are white in color when alive.
Genus. Bonassa
Barnard & Karaman, 1991
7B3E8466-1AF0-5529-8DA4-B003B50327FF
Diagnosis.
Antenna 1 with strong callynophore in male. Antenna 2 flagellum elongate in male. Epistome and upper lip produced. Maxilla 2 inner plate narrow. Gnathopod 1 simple. Gnathopod 2 minutely chelate. Uropod 2 inner ramus with dorsal notch, gradually narrowing distally. Uropod 3 outer ramus 1-articulate. Telson entire.
. Bonassa bonairensis
(Stephensen, 1933)
C69C00FD-769C-5987-8EE7-84C0450CA84D
Figure 2.
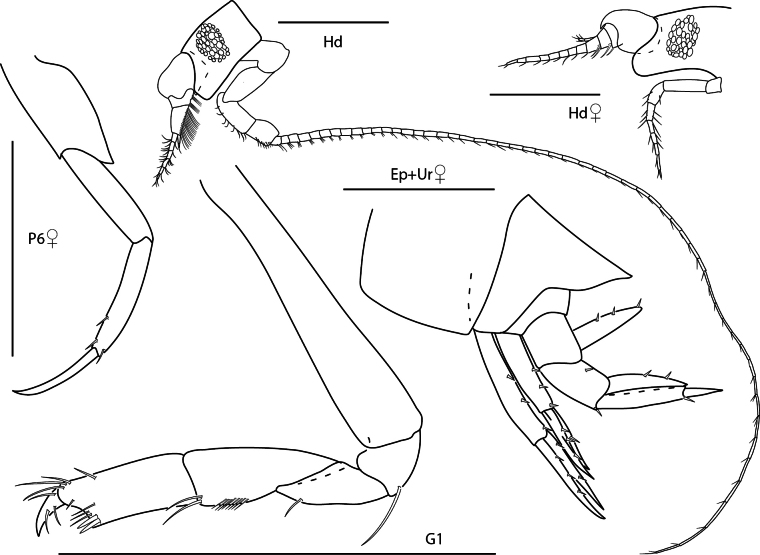
Bonassabonairensis, female, 2.8 mm, pereopod 7, gnathopod 1 lateral, telson, uropod 3, head, epistome and upper lip. Scale bars: 0.5 mm.
Lysianassa (?) bonairensis Stephensen, 1933a: 416–420, figs 1, 2; Stephensen 1948: 1, 3.
Lysianassa bonairensis J.L. Barnard, 1958: 94; Ortiz 1979: 19.
Bonassa bonairensis Barnard & Karaman, 1991: 472; Lowry and Stoddart 1997: 54–58, figs 21–23.
Material examined.
Panama • 2–3 mm • 1 ♀; Bocas del Toro, Swan Cay; 9.4533°N, 82.2983°W; depth 2–3 m, among algae; 4 Aug 2005; S. DeGrave leg.; GCRL 6655 • 1 ♀; Bocas del Toro, Drago; 9.418056°N, 82.3375°W; depth 2–3 m, among coral rubble, 9 Aug 2021; K.N. White leg.; USNM 1739773 • 1 juvenile; Bocas del Toro, Hospital Point; 9.331967°N, 82.214817°W; depth 1–3 m, among coral rubble; 22 June 2023; K.N. White leg.; USNM 1739774.
Diagnosis.
Epistome produced, rounded, subequal to produced upper lip. Antenna 1 with strong callynophore in female. Gnathopod 1 propodus distally narrowing. Pereopod 7 basis greatly expanded, posteriorly rounded; merus greatly expanded, approximately 3 × width of carpus. Uropod 3 rami narrow, apically acute, and lacking plumose setae in female. Telson distal margin truncate, slightly emarginate.
Distribution.
Lesser Antilles: Bonaire Island (Stephensen 1933a; Lowry and Stoddart 1997); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods occur among algae and coral rubble at depths of 1–3 m. Panamanian specimens agree closely with previous descriptions of the species, with the exception of a slightly emarginate telson, with the exception of the uropod 3, which is documented for the first time in a female. This species is easily distinguishable based on the expanded pereopod 7 basis and merus. Panamanian specimens are a translucent white color when alive.
Genus. Concarnes
Barnard & Karaman, 1991
8A342C4D-0B62-5301-80A9-845DBAB2D7A1
Diagnosis.
Antenna 1 with strong callynophore in male, lacking in female. Antenna 2 flagellum short in male and female. Epistome and upper lip produced. Mouthparts forming quadrate bundle. Maxilla 2 inner plate broad. Gnathopod 1 simple. Uropod 2 inner ramus with dorsal notch, gradually narrowing distally. Uropod 3 outer ramus 2-articulate. Telson weakly cleft.
. Concarnes concavus
(Shoemaker, 1933)
F321EAD3-A4FE-54FC-A33A-C98D209F26BB
Figure 3.
Concarnesconcavus, female, 6.0 mm, gnathopod 2 lateral, uropod 2, uropod 3, head, epistome, and upper lip, telson, gnathopod 1 medial. Scale bars: 0.5 mm.
Socarnes concavus Shoemaker, 1933: 247–248, fig. 1; J.L. Barnard 1958: 99; Gurjanova 1962: 304; Ortiz 1979: 19.
Concarnes concavus Barnard & Karaman, 1991: 477; Lowry and Stoddart 1997: 58–63, figs 24–26; LeCroy 2007: 576, fig. 493.
Material examined.
Panama • 5–6 mm • 1 ♀; Bocas del Toro, Crawl Cay; 9.2475°N, 82.1290°W; depth 5 m, among coral rubble; 12 Aug 2021; K.N. White leg.; USNM 1739775 • 1 ♀; Bocas del Toro, Crawl Cay; 9.2460°N, 82.1369°W; depth 1–4 m, among coral rubble; 25 June 2023; K.N. White leg.; USNM 1739776.
Diagnosis.
Head ocular lobe subacute. Epistome produced, rounded, subequal to produced upper lip. Gnathopod 1 basis slender, elongate; propodus distally narrowing. Gnathopod 2 minutely subchelate. Telson partially cleft, lobes apically rounded.
Distribution.
USA: Santee River, South Carolina (LeCroy 2007); off Sapelo and Little Tybee Islands, Georgia (LeCroy 2007); Dry Tortugas (Shoemaker 1933); Gulf of Mexico from Florida Keys to Panama City (Thomas 1993; Lowry and Stoddart 1997; LeCroy 2007); Belize (Thomas 1993); Panama: Bocas del Toro (Miloslavich et al. 2010; present study).
Ecology and remarks.
These amphipods are associated with coral rubble and coarse sand at depths of 1–80 m. Panamanian specimens agree closely with previous descriptions of the species. This species is easily recognizable by the subacute ocular lobe, produced epistome and upper lip, and slender, elongate basis of gnathopod 1. Panamanian specimens have a distinct red coloration on the tips of antennae and on the anterior half of the body and have a white snowflake pattern on the posterior half of the body when alive.
Genus. Lysianopsis
Holmes, 1903
FCB28C7F-BF97-549B-A8BF-7F6E77467DC2
Diagnosis.
Antenna 1 with strong callynophore in male, weak or lacking in female. Antenna 2 flagellum short in male and female. Epistome not produced; upper lip produced. Maxilla 2 inner plate narrow. Gnathopod 1 simple. Gnathopod 2 minutely chelate. Uropod 2 inner ramus with dorsal notch, gradually narrowing distally. Uropod 3 outer ramus 1-articulate. Telson entire.
. Lysianopsis hummelincki
(Stephensen, 1933)
D13FA4B9-0A9A-55C2-830A-AEE4DDE79CCB
Figure 4.
Lysianopsishummelincki, male 4.0 mm, head, upper lip, and epistome, pereopod 7, gnathopod 1, uropod 2, uropod 3. Scale bars: 0.5 mm.
Lysianassa hummelincki Stephensen, 1933b: 438–440, fig. 1; Pirlot 1936: 256; Stephensen 1948: 1, 3, table 1; J.L. Barnard 1958: 94; Hurley 1963: 72; Ortiz 1979: 19.
Lysianassa falcata Stephensen, 1933b: 440–441, fig. 2; Stephensen 1948: 1, 4, table 1; J.L. Barnard 1958: 94; Ortiz 1979: 19.
Lysianopsis alba Barnard & Karaman, 1991: 499 (in part).
Falcanassa falcata Barnard & Karaman, 1991: 486.
Lysianopsis hummelincki Lowry & Stoddart, 1997: 82–89, figs 37–39.
Material examined.
Panama • 4 mm • 1 ♂; Bocas del Toro, Hospital Point; 9.3320°N, 82. 2148°W; depth 1–3 m, among coral rubble; 22 June 2023; K.N. White leg.; USNM 1739777.
Diagnosis.
Upper lip produced well beyond epistome; epistome straight. Gnathopod 1 of male prehensile. Pereopod 7 basis slightly expanded, posterior margin almost straight, merus slightly expanded, approximately 1.4 × width of carpus. Uropod 3 peduncle length about 1.5 × width; outer ramus 1-articulate. Telson distal margin rounded.
Distribution.
Lesser Antilles: Curaçao (Stephensen 1933b); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with sand and coral rubble at depths of intertidal 0–12 m. Panamanian specimens agree closely with previous descriptions with the exception of the almost straight posterior margin on the pereopod 7 basis, which was described by Lowry and Stoddart (1997) as slightly concave. This species is easily recognizable by the 1-articulate outer ramus on uropod 3 and the prehensile gnathopod 1 in males. Panamanian specimens are white with brown spots when alive.
. Lysianopsis ozona
Lowry & Stoddart, 1997
27430BDD-E897-5EF0-8041-A84237D7079E
Figure 5.
Lysianopsisozona, male, 3.2 mm, head, upper lip, and epistome, uropod 2, uropod 3, gnathopod 1 lateral; male, 6.5 mm, maxilla 2, telson. Scale bars: 0.5 mm.
Lysianopsis ozona Lowry & Stoddart, 1997: 87–91, figs 40–42.
Material examined.
Panama • 3.2–8.5 mm • 2 ♀; Bocas del Toro, Bastamientos; depth 0–1 m, mangrove scrapings; 1 Aug 2005; T.A. Haney leg.; GCRL 6656. • 2 ♂; Bocas del Toro, Hospital Bight; 9.3045°N, 82.3160°W; depth 1.5 m, among coral rubble; 7 Aug 2005; T.A. Haney leg.; GCRL 6657 • 1 ♀; Bocas del Toro, Marina Bocas; depth 0–1 m, associated with Phallusianigra ascidian; 5 June 2009; R. Rocha leg.; GCRL 6658 • 1 ♂; Bocas del Toro, Isla Solarte; 9.2901°N, 82.1897°W; depth 1–5 m, associated with solitary ascidian; 8 Aug 2021; K.N. White leg.; USNM 1739778.
Diagnosis.
Epistome concave, subequal to upper lip. Gnathopod 1 propodus posterodistal margin straight; not sexually dimorphic. Uropod 3 peduncle length approximately 1.5 × width; outer ramus 2-articulate. Telson apical margin slightly truncate, apical margin with four short setae medially.
Distribution.
USA: Eastern Gulf of Mexico (Lowry and Stoddart 1997); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with sand, coral rubble, and various invertebrates at depths of 0–29 m. Panamanian specimens agree closely with the description provided by Lowry and Stoddart (1997). This species is easily recognizable by the concave epistome and the short uropod 3 peduncle and 2-articulate outer ramus. Panamanian specimens have an orange-brown coloration with white stripes along the pereonite edges when alive.
Genus. Shoemakerella
Pirlot, 1936
3A5725C0-F262-5615-947C-E602205B0DD2
Diagnosis.
Antenna 1 with weak callynophore in male, lacking in female. Antenna 2 flagellum short in male and female. Epistome not produced; upper lip produced. Maxilla 2 inner plate wider than outer plate. Gnathopod 1 simple. Pereopods 3–4 merus not enlarged compared to carpus. Uropod 2 inner ramus with dorsal notch, abruptly narrowing distally. Uropod 3 outer ramus 1-articulate. Telson entire, dorsal setae inserted proximally (compared to other genera).
. Shoemakerella cubensis
(Stebbing, 1897)
AFED234F-9004-5EE8-8909-B8B1C2506EB1
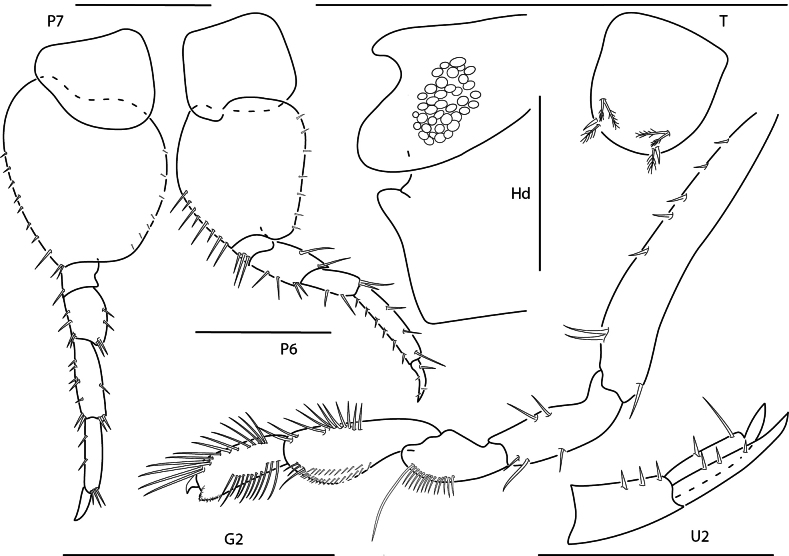
Figure 6.
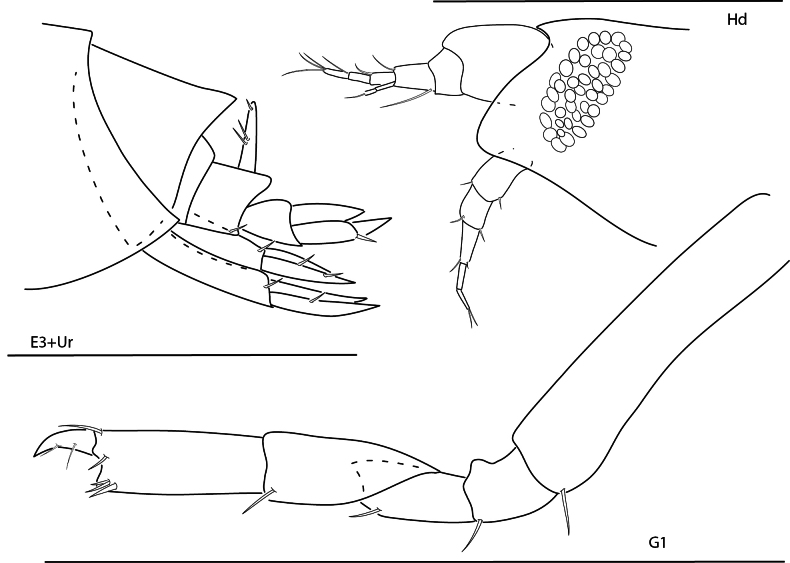
Shoemakerellacubensis, male, 4.0 mm, pereopod 7, pereopod 6, head, epistome, and upper lip, telson, gnathopod 2 lateral, uropod 2. Scale bars: 0.5 mm.
Figure 11.
Photographs of live specimens unless noted AShoemakerellacubensis (ethanol preserved specimen) BShoemakerellalowryiCLepidepecreummagdalenensis (ethanol preserved specimen) DOrchomenellathomasi (ethanol preserved specimen). Scale bars: 1.0 mm.
Lysianax cubensis Stebbing, 1897: 29–30, pl. 7B; Hurley 1963: 70–71, fig. 20 b, c; Lowry and Stoddart 1989: 236–237.
Lysianassa cubensis Stebbing, 1906: 38; Shoemaker 1935: 232–234, fig. 1.
Lysanopsis alba Pearse, 1912: 369, fig. 1 (in part); Shoemaker 1921: 99.
Shoemakerella nasuta Pirlot, 1936: 265–266; Pirlot 1939: 47–48; Shoemaker 1948: 1–2; J.L. Barnard 1969: 180; Ortiz and Lalana Rueda 1993: 26; Ortiz and Lemaitre 1994: 124.
Lysianopsis cubensis Hurley, 1963: fig. 21a.
Lysianassa nasuta Ortiz, 1978: 8; Ortiz 1979: 19; Lalana Rueda and Pérez Moreno 1985: 51; Lalana Rueda et al. 1989: 210; Lalana Rueda and Ortiz 1990: 196; Ortiz and Lalana Rueda 1992: 40.
Shoemakerella cubensis Barnard & Karaman, 1991: 530; Lowry and Stoddart 1997: 92–98, figs 43–45; LeCroy 2007: 588, fig. 495.
Material examined.
Panama • 1.5–4 mm • 3 ♀, 1 juvenile; Bocas del Toro, Hospital Point; 9.3336°N, 82.2188°W; depth 15 m, among coral rubble and Halimeda; 6 Aug 2005; S. DeGrave and M. Salazar leg.; GCRL 6659 • 2 ♂, 9 ♀, 11 juvenile; Bocas del Toro, Lime Point; 9.4149°N, 82.3323°W; depth 0.2–0.5 m, among coral rubble and red algae; 5 Aug 2005; S. DeGrave and M. Salazar leg.; GCRL 6660 • 1 juvenile; Bocas del Toro, Juan Point; 9.3015°N, 82.2940°W; depth 10 m, among coral rubble; 7 Aug 2021; K.N. White leg.; USNM 1739779 • 1 ♂, 2 juvenile; Bocas del Toro, Isla Solarte; 9.29011°N, 82.1897°W; depth 1–5 m, mangrove scrapings; 8 Aug 2021; K.N. White leg.; USNM 1739780, USNM 1739781.
Diagnosis.
Head and body with tiny setules. Epistome strongly concave. Pereopod 6 basis posterior margin nearly straight. Pereopod 7 propodus length ~5 × width. Telson apex rounded.
Distribution.
USA: Panama City to Dry Tortugas, Florida (Lowry and Stoddart 1997; LeCroy 2007); Cuba (Stebbing 1897); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with algae and coral rubble at depths of 2–69 m. Panamanian specimens closely resemble previously described specimens and can be readily distinguished from Shoemakerellalowryi Gable & Lazo-Wasem, 1990 based on the pereopod 6 basis posterior margin, pereopod 7 propodus length relative to the carpus length, and the telson apex. Panamanian specimens are yellow-orange in color when alive.
. Shoemakerella lowryi
Gable & Lazo-Wasem, 1990
E3729DE2-9957-53F4-9ECF-A033CD5DAF0F
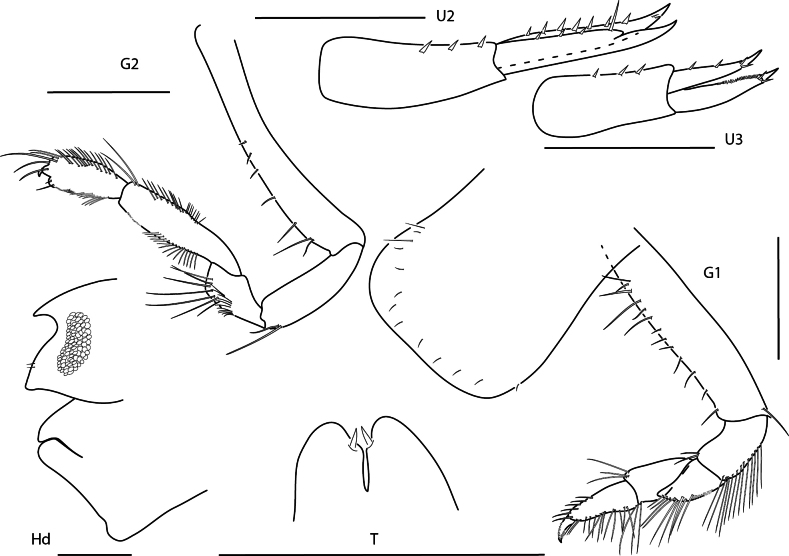
Figure 7.
Shoemakerellalowryi, male, 4.5 mm, pereopod 7, pereopod 6, head, epistome and upper lip, pereopod 3, maxilla 2, telson, gnathopod 1 lateral. Scale bars: 0.5 mm.
Lysianassa punctata Kunkel, 1910: 8–10, fig. 1; Johnson 1986: 377, fig. 124.
Shoemakerella lowryi Gable & Lazo-Wasem, 1990: 727–733, figs 5–7.
Material examined.
Panama • 2–5.5 mm • 1 ♂; Bocas del Toro, San Cristobal; 9.2625°N, 82.2350°W; depth 15 m, among coral rubble; 10 August 2021; K.N. White leg.; USNM 1739782 • 1 ♀; Bocas del Toro, Swan Cay; 9.4536°N, 82.300033°W; depth 2 m, among sponges; 24 Jun 2023; K.N. White leg.; USNM 1739783 • 2 ♀; Bocas del Toro, Crawl Cay; 9.245967°N, 82.136867°W; depth 1–4 m, among coral rubble; 25 June 2023; K.N. White leg.; USNM 1739784 • 4 ♀; Bocas del Toro, Cayo Zapatilla 1; 9.2700°N, 82.0587°W; depth 10–11 m, among coral rubble; 28 June 2023; K.N. White leg.; USNM 1739785.
Diagnosis.
Head and body with tiny setules. Epistome weakly concave. Pereopod 6 basis posterior margin slightly concave. Pereopod 7 propodus length ~9 × width. Telson apex truncate.
Distribution.
Bermuda (Gable and Lazo-Wasem 1990); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with algae, seagrass, and coral rubble at depths of 0.5–9 m. Panamanian specimens closely resemble previously described specimens and can be readily distinguished from Shoemakerellacubensis based on the pereopod 6 basis posterior margin, pereopod 7 propodus length relative to the carpus length, and the telson apex. Panamanian specimens are transparent white in color with brown spots when alive.
Family Tryphosidae Lowry & Stoddart, 1997
Genus. Lepidepecreum
Bate & Westwood, 1868
78CF2976-D981-50B8-9320-26EFDD46B837
Diagnosis.
Antenna 1 with strong callynophore in male and weak callynophore in female. Antenna 2 of male elongate. Antenna 2 peduncular article 3 elongate in male and female. Maxilla 2 inner plate narrow. Gnathopod 1 subchelate; coxa large, about as long as coxa 2; carpus long (length 2 to 4 × width). Uropod 2 inner ramus without distinct dorsal notch. Uropod 3 outer ramus 2-articulate. Telson cleft.
. Lepidepecreum cf. magdalenensis
(Shoemaker, 1942)
32B66617-36BB-5AC2-B3AA-F83D1595E1BB
Figure 8.
Lepidepecreummagdalenensis, female, 3.0 mm, head, epimeron 3 and urosome, pereopod 6; male, 2.8 mm, head, gnathopod 1 lateral. Scale bars: 0.5 mm.
Orchomenella magdalenensis Shoemaker, 1942: 4–7, fig. 1.
Lepidepecreum magdalenensis Lowry & Stoddart, 2002: 173–174; LeCroy 2007: 580, fig. 492.
Material examined.
Panama • 2–3 mm • 6 ♂, 16 ♀; Bocas del Toro, Drago Beach; 9.4172°N, 82.3248°W; depth 0–1 m, in sand; 27 June 2023; K.N. White leg.; USNM 1739786.
Diagnosis.
Head ocular lobe subrectangular. Gnathopod 1 carpus as long as propodus. Epimeron 3 posteroventral corner subquadrate. Urosomite 1 with dorsodistally acute carina. Uropod 3 inner ramus with two marginal spines.
Distribution.
USA: Pacific California (Shoemaker 1942); Florida from Cape Romano to the lower Florida Keys (LeCroy, 2007); Cuba? (Ortiz 1978); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with sand at depths of 0.5–27 m. Panamanian specimens closely resemble previously described specimens, except for a weak callynophore in females (strong in original description) and uropod 3 inner ramus having 2 marginal spines (3 in original description). LeCroy (2007) notes that Florida specimens of L.cfmagdalenensis have only one spine, suggesting that this may vary among specimens of this genus. The weak callynophore on antenna 1 of females may suggest that L.magdalenensis represents a species complex, but this can only be resolved with further examination of all collections. Panamanian specimens are white in color when alive.
Genus. Orchomenella
Sars, 1890
0E825C08-58EE-5A37-8EC0-47A44A2C149E
Diagnosis.
Antenna 2 of male flagellum elongate. Antenna 2 peduncular article 3 short. Maxilla 2 inner plate narrow. Gnathopod 1 subchelate; carpus short (length less than 2 × width). Uropod 2 inner ramus without distinct dorsal notch. Telson cleft.
. Orchomenella thomasi
Lowry & Stoddart, 1997
5477E6BE-8893-5CF9-B18C-DA17F10A7295
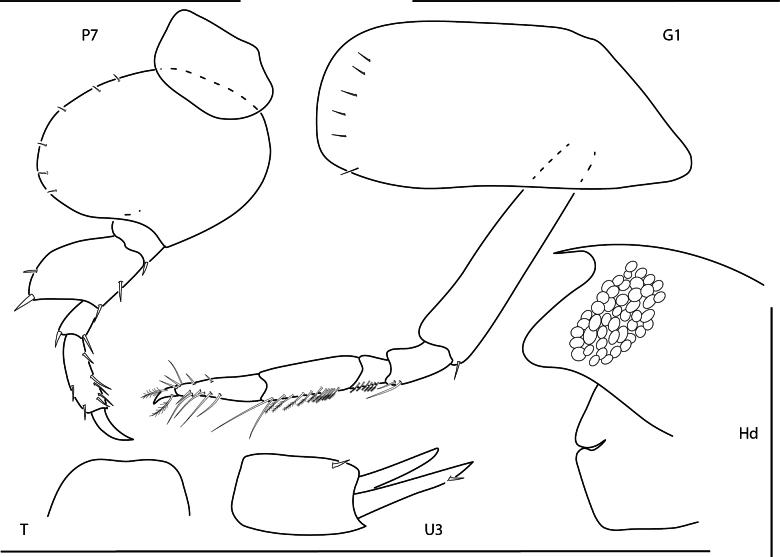
Figure 9.
Orchomenellathomasi, female, 1.5 mm, head, epimeron 3 and urosome, gnathopod 1 lateral. Scale bars: 0.5 mm.
Orchomenella thomasi Lowry & Stoddart, 1997: 109–113, figs 52–53; LeCroy 2007: 586, fig. 502.
Material examined.
Panama • 1.5 mm • 1 ♀; Bocas del Toro, Cayo Zapatilla 1; 9.2700°N, 82.0587°W; depth 10–11 m, among coral rubble; 28 June 2023; K.N. White leg.; USNM 1739787.
Diagnosis.
Head ocular lobe subtriangular. Gnathopod 1 carpus shorter than propodus. Epimeron 3 posteroventral corner acute. Urosomite 1 with dorsodistally acute carina. Uropod 3 inner ramus bare; outer ramus 2-articulate.
Distribution.
USA: from Sanibel Island, Florida to Louisiana (Lowry and Stoddart 1997; LeCroy 2007); Panama: Bocas del Toro (present study).
Ecology and remarks.
These amphipods are associated with sand and coral rubble at depths of 10–73 m. Panamanian specimens closely resemble previously described specimens. Panamanian specimens are white in color when alive.
Identification Key to the Caribbean Lysianassidira of Panama
| 1 | Eye absent; pereopod 5 basis narrowly expanded | 2 |
| – | Eye present, well developed; pereopod 5 basis broadly expanded | 3 |
| 2 | Head ocular lobe produced; epimeron 3 posteroventral margin with acute tooth; telson deeply cleft, about 75% | Paracentromedoncarabicus |
| – | Head ocular lobe evenly rounded; epimeron 3 posteroventral margin rounded; telson shallowly cleft, less than 50% | Vemanacompressa |
| 3 | Gnathopod 1 subchelate; urosomite 1 with dorsodistally acute carina; uropod 2 inner ramus without distinct dorsal notch (Figs 8, 9) | 4 |
| – | Gnathopod 1 simple; urosomite 1 without dorsodistal carina; uropod 2 inner ramus with distinct dorsal notch (Fig. 1) | 5 |
| 4 | Antenna 2 peduncle article 3 long in female; head ocular lobe subrectangular; gnathopod 1 carpus as long as propodus; epimeron 3 posteroventral corner subquadrate; uropod 3 inner ramus with marginal spines (Fig. 8) | Lepidepecreummagdalenensis |
| – | Antenna 2 peduncle article 3 short in female; head ocular lobe subtriangular; gnathopod 1 carpus shorter than propodus; epimeron 3 posteroventral corner acute; uropod 3 inner ramus bare (Fig. 9) | Orchomenellathomasi |
| 5 | Gnathopod 1 dactylus reduced, complex, covered in long, slender cuticular teeth; telson entire | Eclecticuseclecticus |
| – | Gnathopod 1 dactylus not reduced, simple; telson entire or partially cleft | 6 |
| 6 | Gnathopod 2 minutely subchelate; telson partially cleft (Fig. 3) | Concarnesconcavus |
| – | Gnathopod 2 minutely chelate (Figs 5, 6); telson entire (Figs 1, 6) | 7 |
| 7 | Maxilla 2 inner plate wider than outer plate (Fig. 7); uropod 2 abruptly narrowing at notch (Fig. 6 | 8 |
| – | Maxilla 2 inner plate narrow, similar in width to outer plate (Fig. 5); uropod 2 gradually narrowing at notch (Fig. 1) | 9 |
| 8 | Pereopod 6 basis posterior margin nearly straight; pereopod 7 propodus length ~5 × width; telson apex rounded (Fig. 6) | Shoemakerellacubensis |
| – | Pereopod 6 basis posterior margin slightly concave; pereopod 7 propodus length ~9 × width; telson apex truncate (Fig. 7) | Shoemakerellalowryi |
| 9 | Epistome rounded; uropod 3 outer ramus 1-articulate (Fig. 2) | 10 |
| – | Epistome concave; uropod 3 outer ramus 2-articulate (Fig. 1) | 11 |
| 10 | Epistome produced, subequal to produced upper lip; gnathopod 1 basis slender; pereopod 7 basis greatly expanded, posteriorly rounded, merus greatly expanded, approximately 3 × width of carpus (Fig. 2) | Bonassabonairensis |
| – | Epistome not produced, upper lip produced; gnathopod 1 basis stout; pereopod 7 basis slightly expanded, posterior margin almost straight, merus slightly expanded, approximately 1.4 × width of carpus (Fig. 4) | Lysianopsishummelincki |
| 11 | Upper lip projecting well beyond epistome; gnathopod 1 propodus posterodistal margin slightly concave; uropod 3 peduncle long, length at least 2 × width; telson apical margin slightly emarginate (Fig. 1) | Arugaholmesi |
| – | Upper lip subequal to epistome; gnathopod 1 propodus posterodistal margin straight; uropod 3 peduncle short, length approximately 1.5 × width; telson apical margin slightly truncate (Fig. 5) | Lysianopsisozona |
Discussion
The results of this study represent range extensions for eight species of lysianassid amphipods to include the Caribbean waters of Panama. One species collected in this study, Concarnesconcavus, has been recorded from the Caribbean of Panama by Miloslavich et al. (2010), yet those authors did not provide any specific locality information, so it is unclear what the exact range of this species is in the Caribbean waters of Panama. Two species documented here have a distribution pattern spanning the eastern Pacific and western Caribbean (Arugaholmesi and Lepidepecreummagdalenensis). These distribution patterns may suggest that the species were established more than 3 mya, before the isthmus of Panama closed, or that we have species complexes that need to be investigated further.
Characters that have been used to identify lysianassid amphipods in the past, such as setae patterns on the dorsal surface of the body appear to be variable in Panamanian specimens and should not be used for identification. Sexual dimorphism is also used frequently but can be problematic when you have only one specimen or gender. Mouthparts are also often used as diagnostic characters which can be difficult for non-experts; thus, I included as many other characters as possible in this identification key.
Supplementary Material
Acknowledgements
Logistical support and facilities were provided by Georgia College & State University Department of Biological and Environmental Sciences and the Smithsonian Tropical Research Institute (STRI). Special thanks to Carolina Cesar and Valentina Cardona for assistance with diving and collecting in Bocas del Toro. The author also wishes to thank Dr Lauren Hughes and the Amphipod Taxonomy Course members for collecting assistance in 2023. Special thanks go to Sara LeCroy for her loan of specimens collected in 2005 and to Sally Sir, Sara LeCroy, and Tammy Horton for suggestions to improve the manuscript.
Citation
White KN (2024) Caribbean Amphipoda (Crustacea) of Panama. Part III: parvorder Lysianassidira. ZooKeys 1216: 149–171. https://doi.org/10.3897/zookeys.1216.135258
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
Funding for this study was provided by a National Science Foundation grant: Collaborative Research: ARTS: Understanding Tropical Invertebrate Diversity Through Integrative Revisionary Systematics and Training (1856421).
Author contributions
Conceptualization: KNW. Data curation: KNW. Formal analysis: KNW. Funding acquisition: KNW. Investigation: KNW. Methodology: KNW. Project administration: KNW. Writing - original draft: KNW. Writing - review and editing: KNW.
Author ORCIDs
Kristine N. White https://orcid.org/0000-0002-5203-1656
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information.
Supplementary materials
Locality table
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Author: Kristine N. White
Data type
xlsx
References
- Andres HG. (1977) Gammaridea (Crustacea, Amphipoda) aus dem Iberischen Tiefseebecken Auswertung des materials der Fahrten 3 und 15 von F. S. Meteor. Meteor Forschungsergebnisse (Reihe D) 25: 54–67. [Google Scholar]
- Austin WC. (1985) An Annotated Checklist of Marine Invertebrates in the Cold Temperate Northeast Pacific. Cowichan, B. C., Khoyatan Marine Laboratory, 682 pp. [Google Scholar]
- Barnard JL. (1955) Notes on the amphipod genus Aruga with the description of a new species. Bulletin of the Southern California Academy of Sciences 54(2): 97–103. [Google Scholar]
- Barnard JL. (1958) Index to the families, genera, and species of the gammaridean Amphipoda (Crustacea). Allan Hancock Foundation Publications, Occasional Paper 19: 1–145. [Google Scholar]
- Barnard JL. (1959) Estuarine Amphipoda. Allan Hancock Foundation Publications, Occasional Paper 21: 13–9. [Google Scholar]
- Barnard JL. (1961) Gammaridean Amphipoda from depths of 400 to 6000 meters. Galathea Report 5: 23–128. [Google Scholar]
- Barnard JL. (1964) Deep-sea Amphipoda (Crustacea) collected by the R/V “Vema” in the eastern Pacific Ocean and the Caribbean and Mediterranean seas. Bulletin of the American Museum of Natural History 127(1): 1–46. [Google Scholar]
- Barnard JL. (1966a) Benthic Amphipoda of Monterey Bay, California. Proceedings of the United States National Museum 119: 1–41. 10.5479/si.00963801.119-3541.1 [DOI] [Google Scholar]
- Barnard JL. (1966b) Submarine canyons of southern California. Part V. Systematics: Amphipoda. Allan Hancock Pacific Expeditions 27(5): 1–166. [Google Scholar]
- Barnard JL. (1969) Gammaridean Amphipoda of the rocky intertidal of California: Monterey Bay to La Jolla. United States National Museum Bulletin 258: 1–230. 10.5479/si.03629236.258.1 [DOI] [Google Scholar]
- Barnard JL. (1979) Littoral gammaridean Amphipoda from the Gulf of California and the Galapagos Islands. Smithsonian Contributions to Zoology 271: 1–149. 10.5479/si.00810282.271 [DOI] [Google Scholar]
- Barnard JL, Karaman GS. (1991) The families and genera of marine gammaridean Amphipoda (Except marine Gammaroidea). Records of the Australian Museum Supplement 13 (Part 2): 419–866. 10.3853/j.0812-7387.13.1991.367 [DOI]
- Bate CS, Westwood JO. (1868) A History of the British sessile-eyed Crustacea. Vol. 11. John Van Voorst, London, 401–536.
- Coleman CO. (2003) “Digital inking”: how to make perfect line drawings on computers. Organisms Diversity & Evolution 3(14): 1–14. 10.1078/1439-6092-00081 [DOI] [Google Scholar]
- Dana JD. (1849) Synopsis of the genera of Gammaracea. American Journal of Science and Arts, Series 2, 8: 135–140.
- Dana JD. (1852) On the classification of the CrustaceaChoristopoda or Tetradecapoda. The American Journal of Science and Arts, Second Series 14(41): 297–316. [Google Scholar]
- Gable MF, Lazo-Wasem EA. (1990) Lysianassidae (Amphipoda: Lysianassoidea) of Bermuda. Journal of Crustacean Biology 10(4): 721–734. 10.2307/1548416 [DOI] [Google Scholar]
- Gurjanova EF. (1962) Amphipoda of the northern part of the Pacific Ocean (Amphipoda-Gammaridea). Part 1. Akademiya Nauk SSSR, Opredeliteli po Faune SSSR 74: 1–440. [Google Scholar]
- Holmes SJ. (1903) Synopses of North-American invertebrates. XVIII. The Amphipoda. American Naturalist 37: 267–292. 10.1086/278286 [DOI] [Google Scholar]
- Holmes SJ. (1908) The Amphipoda collected by the U.S. Bureau of Fisheries steamer Albatross off the west coast of North America, in 1903 and 1904, with descriptions of a new family and several new genera and species. Proceedings of the United States National Museum 35: 489–543. 10.5479/si.00963801.35-1654.489 [DOI] [Google Scholar]
- Horton T, Lowry J, De Broyer C, Bellan-Santini D, Copilaş-Ciocianu D, Corbari L, Costello MJ, Daneliya M, Dauvin J-C, Fišer C, Gasca R, Grabowski M, Guerra-García JM, Hendrycks E, Hughes L, Jaume D, Jazdzewski K, Kim Y-H, King R, Krapp-Schickel T, LeCroy S, Lörz A-N, Mamos T, Senna AR, Serejo C, Souza-Filho JF, Tandberg AH, Thomas JD, Thurston M, Vader W, Väinölä R, Valls Domedel G, Vonk R, White K, Zeidle W. (2024) World Amphipoda Database. [on 2024-07-05] 10.14284/368 [DOI]
- Hurley DE. (1963) Amphipoda of the family Lysianassidae from the west coast of North and Central America. Allan Hancock Foundation Publications, Occasional Paper 25: 1–160. [Google Scholar]
- Johnson SE. (1986) Order Amphipoda. In: Sterrer W. (Ed.) Marine Fauna and Flora of Bermuda.John Wiley & Sons, New York, 372–381.
- Kunkel BW. (1910) The Amphipoda of Bermuda. Transactions of the Connecticut Academy of Arts and Sciences 16: 1–116. [Google Scholar]
- Lalana Rueda R, Ortiz M. (1990) Zoobentos de Cayo Hicacos, SE de la Isla de la Juventud, Cuba. Revista de Investigaciones Marinas 11(3): 191–199. [Google Scholar]
- Lalana Rueda R, Pérez Moreno M. (1985) Estudio cualitativo y cuantitativo de la fauna asociada a las raices de Rhizophoramangle en la cayería este de la Isla de la Juventud. Revista Investigaciones Marinas 6(2–3): 45–57. [Google Scholar]
- Lalana Rueda R, Capetillo R, Brito E, Diaz E, Cruz R. (1989) Estudio del zoobentos asociado a Laurenciaintricata en un área de juveniles de langosta, al SE de la Isla de la Juventud, Cuba. Revista Investigaciones Marinas 10(3): 207–218. [Google Scholar]
- LeCroy SE. (2007) An Illustrated Identification Guide to the Nearshore Marine and Estuarine Gammaridean Amphipoda of Florida Volume 4: Families Anamixidae, Eusiridae, Hyalellidae, Hyalidae, Iphimediidae, Ischyroceridae, Lysianassidae, Megaluropidae, and Melphidippidae. Florida Department of Environmental Protection Annual Report Contract No. WM724 1: 1–195.
- LeCroy SE, Gasca R, Winfield I, Ortiz M, Escobar-Briones E. (2009) Amphipoda (Crustacea) of the Gulf of Mexico. In: Felder DL, Camp DK. (Eds) Gulf of Mexico: Origin, Waters, and Biota.Texas A&M University Press, Texas, 941–972.
- Lilljeborg W. (1865) Bidrag till kannedomen om underfamiljen Lysianassina inom underordningen Amphipoda bland kraftdjuren. Nova Acta Regiae Societatis Scientarum Upsaliensis 3: 1–25. [Google Scholar]
- Lowry JK. (2008) Alicellidae and Valettiopsidae, two new callynophorate families (Crustacea, Amphipoda). Zootaxa 1843: 57–66. 10.11646/zootaxa.1843.1.5 [DOI] [Google Scholar]
- Lowry JK, Myers AA. (2017) A Phylogeny and Classification of the Amphipoda with the establishment of the new order Ingolfiellida (Crustacea: Peracarida). Zootaxa 4265(1): 1–89. 10.11646/zootaxa.4265.1.1 [DOI] [PubMed] [Google Scholar]
- Lowry JK, Stoddart HE. (1989) Shoemakerella Pirlot, 1936 (Crustacea, Amphipoda): proposed designation of Lysianaxcubensis Stebbing, 1897, as type species. Bulletin of Zoological Nomenclature 46(4): 236–238. 10.5962/bhl.part.550 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (1990) The Wandinidae, a new indo-pacific family of Lysianassoid Amphipoda (Crustacea). Records of the Australian Museum 42: 159–171. 10.3853/j.0067-1975.42.1990.113 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (1995) The Amphipoda (Crustacea) of Madang Lagoon: Lysianassidae, Opisidae, Uristidae, Wandinidae and Stegocephalidae. In: Lowry JK. (Ed.) Amphipoda (Crustacea) of the Madang Lagoon, Papua New Guinea.Records of the Australian Museum, Supplement 22: 97–174. 10.3853/j.0812-7387.22.1995.122 [DOI]
- Lowry JK, Stoddart HE. (1996) New lysianassoid amphipod species from Namibia and Madagascar (Lysianassidae Dana, 1849 and Podoprionidae fam. nov.). Bolletino del Museo Civico di Storia Naturale di Verona. 20(1): 225–247. [Google Scholar]
- Lowry JK, Stoddart HE. (1997) AmphipodaCrustacea IV. Families Aristiidae, Cyphocarididae, Endevouridae, Lysianassidae, Scopelocheiridae, Uristidae. Memoirs of the Hourglass Cruises 10: 1–148. [Google Scholar]
- Lowry JK, Stoddart HE. (2002) The Amaryllididae of Australia (Crustacea: Amphipoda: Lysianassoidea). Records of the Australian Museum 54(2): 129–214. 10.3853/j.0067-1975.54.2002.1363 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2010a) The deep-sea scavenging genus Hirondellea (Crustacea: Amphipoda: Lysianassoidea: Hirondelleidae fam. nov.) in Australian waters. Zootaxa 2329: 37–55. 10.11646/zootaxa.2329.1.3 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2010b) Sophrosynidae, a new family in the Lysianassoidea (Crustacea: Amphipoda) with a revision of the genus Sophrosyne. Zootaxa 2370(1): 1–35. 10.11646/zootaxa.2370.1.1 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2010c) The family Izinkalidae fam. nov. (Crustacea: Amphipoda: Lysianassoidea) in Australian waters. Zootaxa 2532(1): 64–68. 10.11646/zootaxa.2532.1.3 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2010d) Kergueleniidae fam. nov. (Crustacea: Amphipoda: Lysianassoidea) in Australian waters. Zootaxa 2564(1): 1–30. 10.11646/zootaxa.2564.1.1 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2011) The new deep-sea families Cebocaridae fam. nov., Cyclocaridae fam. nov. and Thoriellidae fam. nov. (Crustacea: Amphipoda: Lysianassoidea). Zootaxa 2747: 53–68. 10.11646/zootaxa.2747.1.4 [DOI] [Google Scholar]
- Lowry JK, Stoddart HE. (2012) Australian and South African conicostomatine amphipods (Amphipoda: Lysianassoidea: Lysianassidae: Conicostomatinae subfam. nov.). Zootaxa 3248: 43–65. 10.11646/zootaxa.3248.1.4 [DOI] [Google Scholar]
- Martín A, Díaz Y, Miloslavich P, Escobar-Briones E, Guerra-García JM, Ortiz M, Valencia B, Giraldo A, Klein E. (2013) Regional diversity of Amphipoda in the Caribbean Sea. Revista de Bilogía Tropical 61(4): 1681–1720. 10.15517/rbt.v61i4.12816 [DOI] [PubMed] [Google Scholar]
- Miloslavich P, Díaz JM, Klein E, Alvarado JJ, Díaz C, Gobin J, Escobar-briones E, Cruz-motta JJ, Weil E, Cortés J, Bastidas AC, Robertson R, Zapata F, Martín A, Castillo J, Kazandjian A, Ortiz M. (2010) Marine biodiversity in the Caribbean: regional estimates and distribution patterns. PLoS ONE 5(8): e11916. 10.1371/journal.pone.0011916 [DOI] [PMC free article] [PubMed]
- Ortiz M. (1978) Invertebrados marinos bentosicos de Cuba. I. Crustacea, Amphipoda, Gammaridea. Ciencias 38: 3–10. [Google Scholar]
- Ortiz M. (1979) Lista de especes y bibliografía de los anfípodos (Crustacea: Amphipoda) del Mediterráneo Americano. Ciencias (La Habana), Series 8, Investigaciones Marinas 43: 1–40.
- Ortiz M, Lalana Rueda R. (1992) Parasites de anfípodos (Gammaridea) de Cuba. Revista Investigaciones Marinas 13(1): 39–48. [Google Scholar]
- Ortiz M, Lalana Rueda R. (1993) Adición a la lista de especies y bibliografia de los anfípodos (Crustacea: Amphipoda) del Mediterráneo Americano. Revista Investigaciones Marinas 14(1): 16–37. [Google Scholar]
- Ortiz M, Lemaitre R. (1994) Crustaceos Anfipodos (Gammaridea) Colectados en Las Costas del Caribe Colombiano, al sur de Cartagena. Anales del Instituto de Investigaciones Marinas de Punta de Betin 23: 119–127. [Google Scholar]
- Pearse AS. (1912) Notes on certain amphipods from the Gulf of Mexico, with descriptions of new genera and new species. Proceedings of the United States National Museum 43: 369–379. 10.5479/si.00963801.43-1936.369 [DOI] [Google Scholar]
- Pirlot JM. (1936) Les amphipodes de l’expédition du Siboga. Deuxième partie: Les amphipodes gammarides, II. - Les amphipodes de la mer profonde. 3: Addendum et partie générale. III. - Les amphipodes littoraux. 1: Lysianassidae, Ampeliscidae, Leucothoidae, Stenothoidae, Phliantidae, Colomastigidae, Ochlesidae, Liljeborgiidae, Oedicerotidae, Synopiidae, Eusiridae, Gammaridae. Siboga-Expeditie, Monographie 33e: 237–328.
- Pirlot JM. (1939) Amphipoda. Résultats scientifiques des croisières du navire-ecole Belge ‘Mercator’. Mémoires du Musée Royal d’Histoire Naturelle de Belgique, Series 2, 15: 47–80.
- Sars GO. (1890) An Account of the Crustacea of Norway, with Short Descriptions and Figures of all the Species. Vol. I. Amphipoda. Parts 1–3. Christiana, Alb. Cammermeyer, 68 pp. [Google Scholar]
- Shoemaker CR. (1921) Report on the amphipods collected by the Barbados-Antigua Expedition from the University of Iowa in 1918. University of Iowa Studies in Natural History 9(5): 99–102. [Google Scholar]
- Shoemaker CR. (1933) Amphipoda from Florida and the West Indies. American Museum Novitates 598: 1–24. [Google Scholar]
- Shoemaker CR. (1935) The amphipods of Porto Rico and the Virgin Islands. Scientific Survey of Porto Rico and the Virgin Islands (New York Academy of Sciences) 15: 229–253. [Google Scholar]
- Shoemaker CR. (1942) Amphipod crustaceans collected on the Presidential Cruise of 1938. Smithsonian Miscellaneous Collections 101(11): 1–52. [Google Scholar]
- Shoemaker CR. (1948) The Amphipoda of the Smithsonian-Roebling Expedition to Cuba in 1937. Smithsonian Miscellaneous Collections 110(3): 1–15. 10.5962/bhl.part.9028 [DOI] [Google Scholar]
- Stebbing TRR. (1888) Report on the Amphipoda collected by H.M.S. Challenger during the years 1873–1876. Zoology 29: 1–1737. [Google Scholar]
- Stebbing TRR. (1897) Amphipoda from the Copenhagen Museum and other sources. Transactions of the Linnean Society, London, Series 2, Zoology 7: 25–45 [pls 6–14]. 10.1111/j.1096-3642.1897.tb00400.x [DOI]
- Stebbing TRR. (1906) Amphipoda I. Gammaridea. Das Tiereich 21: 1–806. [Google Scholar]
- Stephensen K. (1933a) Zoologische Ergebnisse einer Reise nach Bonaire, Curaçao und Aruba im Jahre 1930. No. 8. Fresh- and brackish-water Amphipoda from Bonaire, Curaçao and Aruba. Zoologische Jahrbucher. Abteilung fur Systematik, 0kologie un Geographie der Tiere 64(3/5): 415–436.
- Stephensen K. (1933b) Zoologische Ergebnisse einer Reise nach Bonaire, Curaçao und Aruba im Jahre 1930. No. 9. Amphipoda from the marine salines of Bonaire and Curaçao. Zoologische Jahrbucher. Abteilung fur Systematik, Okologie un Geographie der Tiere 64(3/5): 437–446.
- Stephensen K. (1948) Amphipods from Curaçao, Bonaire, Aruba and Margarita. Studies on the Fauna of Curaçao, Aruba, Bonaire and the Venezuelan Islands 3(11): 1–20. [Google Scholar]
- Stepien CA, Brusca RC. (1985) Nocturnal attacks on nearshore fishes in southern California by crustacean zooplankton. Marine Ecology Progress Series 25: 91–105. 10.3354/meps025091 [DOI] [Google Scholar]
- Stoddart HE, Lowry JK. (2004) The deep-sea lysianassoid genus Eurythenes (Crustacea, Amphipoda, Eurytheneidae n. fam.). Zoosystema 26(3): 425–468. [Google Scholar]
- Stoddart HE, Lowry JK. (2010) Lepidepecreellidae fam. nov. (Crustacea: Amphipoda: Lysianassoidea) in Australian waters. Zootaxa 2634: 63–68. 10.11646/zootaxa.2634.1.5 [DOI] [Google Scholar]
- Stoddart HE, Lowry JK. (2012) Revision of the lysianassoid genera Acidostoma and Shackletonia (Crustacea: Amphipoda: Acidostomatidae fam.nov.). Zootaxa 3307: 1–34. 10.11646/zootaxa.3307.1.1 [DOI] [Google Scholar]
- Stretch JJ. (1985) Quantitative sampling of demersal zooplankton: reentry and airlift dredge sample comparisons. Journal of Experimental Marine Biology and Ecology 91: 125–136. 10.1016/0022-0981(85)90225-4 [DOI] [Google Scholar]
- Thomas JD. (1993) Identification manual for marine Amphipoda (Gammaridea): I. Common coral reef and rocky bottom amphipods of South Florida. Florida Department of Environmental Protection Final Report Contract No. SP 290: 1–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locality table
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Author: Kristine N. White
Data type
xlsx
Data Availability Statement
All of the data that support the findings of this study are available in the main text or Supplementary Information.