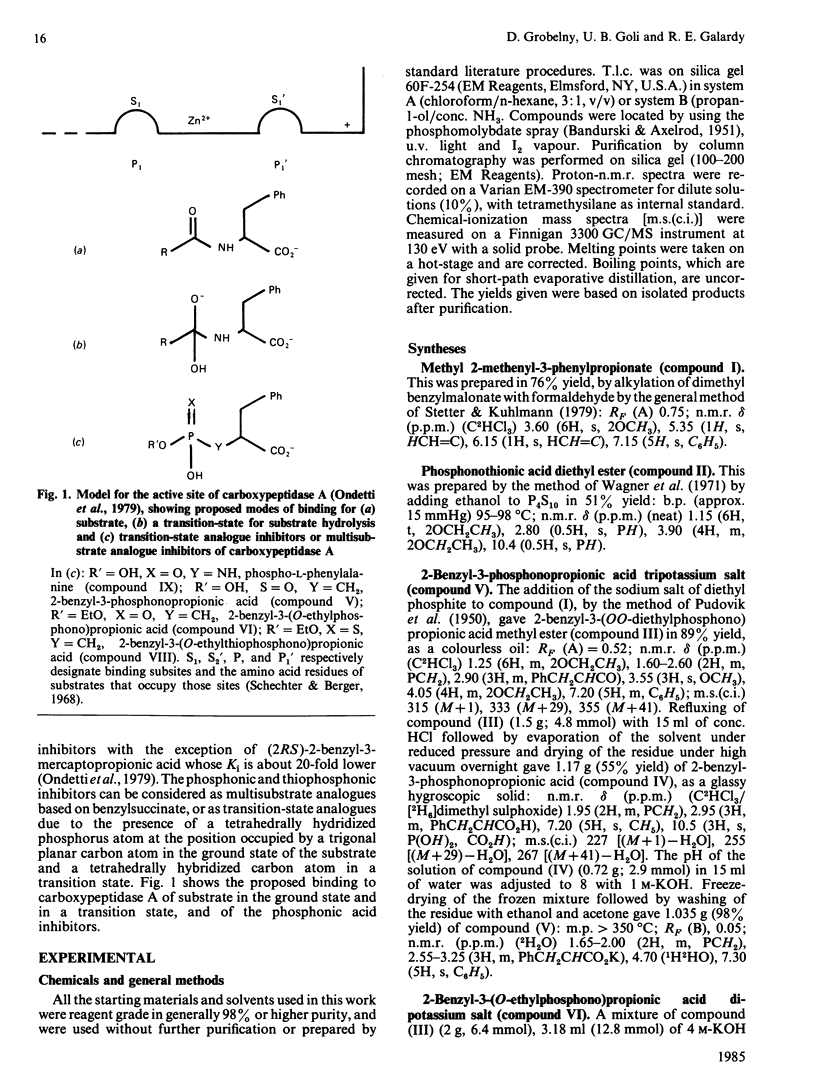
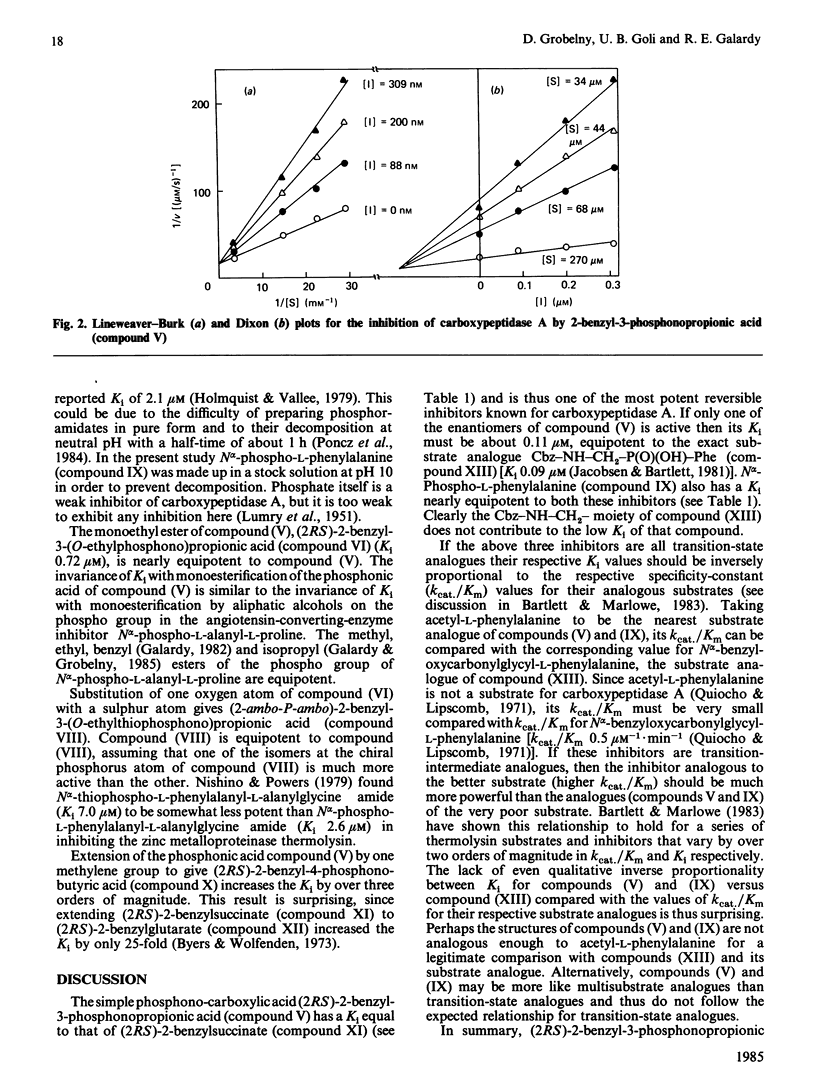
Abstract
A series of phosphonic acid analogues of 2-benzylsuccinate were tested as inhibitors of carboxypeptidase A. The most potent of these, (2RS)-2-benzyl-3-phosphonopropionic acid, had a Ki of 0.22 +/- 0.05 microM, equipotent to (2RS)-2-benzylsuccinate and thus one of the most potent reversible inhibitors known for this enzyme. Lengthening by one methylene group to (2RS)-2-benzyl-4-phosphonobutyric acid increased the Ki to 370 +/- 60 microM. The monoethyl ester (2RS)-2-benzyl-3-(O-ethylphosphono)propionic acid was nearly as potent as (2RS)-2-benzyl-3-phosphonopropionic acid, with a Ki of 0.72 +/- 0.3 microM. The sulphur analogue of the monoethyl ester, 2-ambo-P-ambo-2-benzyl-3-(O-ethylthiophosphono)propionic acid, had a Ki of 2.1 +/- 0.6 microM, nearly as active as (2RS)-2-benzyl-3-(O-ethylphosphono)propionic acid. These phosphonic acids and esters could be considered to be multisubstrate inhibitors of carboxypeptidase A by virtue of their structural analogy with 2-benzylsuccinate. Alternatively, the tetrahedral hybridization at the phosphorus atom suggests that they could be mimicking a tetrahedral transition state for the enzyme-catalysed hydrolysis of substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Bartlett P. A., Marlowe C. K. Phosphonamidates as transition-state analogue inhibitors of thermolysin. Biochemistry. 1983 Sep 27;22(20):4618–4624. doi: 10.1021/bi00289a002. [DOI] [PubMed] [Google Scholar]
- Bolognesi M. C., Matthews B. W. Binding of the biproduct analog L-benzylsuccinic acid to thermolysin determined by X-ray crystallography. J Biol Chem. 1979 Feb 10;254(3):634–639. [PubMed] [Google Scholar]
- Byers L. D., Wolfenden R. Binding of the by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry. 1973 May 22;12(11):2070–2078. doi: 10.1021/bi00735a008. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Grobelny D. Inhibition of collagenase from Clostridium histolyticum by phosphoric and phosphonic amides. Biochemistry. 1983 Sep 13;22(19):4556–4561. doi: 10.1021/bi00288a032. [DOI] [PubMed] [Google Scholar]
- Galardy R. E. Inhibition of angiotensin converting enzyme by phosphoramidates and polyphosphates. Biochemistry. 1982 Nov 9;21(23):5777–5781. doi: 10.1021/bi00266a008. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Kortylewicz Z. P. Inhibition of carboxypeptidase A by aldehyde and ketone substrate analogues. Biochemistry. 1984 Apr 24;23(9):2083–2087. doi: 10.1021/bi00304a032. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Kortylewicz Z. P. Inhibitors of angiotensin-converting enzyme containing a tetrahedral arsenic atom. Biochem J. 1985 Mar 1;226(2):447–454. doi: 10.1042/bj2260447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Metal-coordinating substrate analogs as inhibitors of metalloenzymes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6216–6220. doi: 10.1073/pnas.76.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam C. M., Nishino N., Powers J. C. Inhibition of thermolysin and carboxypeptidase A by phosphoramidates. Biochemistry. 1979 Jul 10;18(14):3032–3038. doi: 10.1021/bi00581a019. [DOI] [PubMed] [Google Scholar]
- Monzingo A. F., Matthews B. W. Structure of a mercaptan-thermolysin complex illustrates mode of inhibition of zinc proteases by substrate-analogue mercaptans. Biochemistry. 1982 Jul 6;21(14):3390–3394. doi: 10.1021/bi00257a022. [DOI] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Design of potent reversible inhibitors for thermolysin. Peptides containing zinc coordinating ligands and their use in affinity chromatography. Biochemistry. 1979 Oct 2;18(20):4340–4347. doi: 10.1021/bi00587a012. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Condon M. E., Reid J., Sabo E. F., Cheung H. S., Cushman D. W. Design of potent and specific inhibitors of carboxypeptidases A and B. Biochemistry. 1979 Apr 17;18(8):1427–1430. doi: 10.1021/bi00575a006. [DOI] [PubMed] [Google Scholar]
- Poncz L., Gerken T. A., Dearborn D. G., Grobelny D., Galardy R. E. Inhibition of the elastase of Pseudomonas aeruginosa by N alpha-phosphoryl dipeptides and kinetics of spontaneous hydrolysis of the inhibitors. Biochemistry. 1984 Jun 5;23(12):2766–2772. doi: 10.1021/bi00307a036. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968 Sep 6;32(5):898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Suh J., Kaiser E. T. pH dependence of the nitrotyrosine-248 and arsanilazotyrosine-248 carboxypeptidase A catalyzed hydrolysis of O-(trans-p-chlorocinnamoyl)-L-beta-phenyllactate. J Am Chem Soc. 1976 Mar 31;98(7):1940–1947. doi: 10.1021/ja00423a048. [DOI] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Matthews B. W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J Mol Biol. 1977 Jul;114(1):119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]


