Abstract
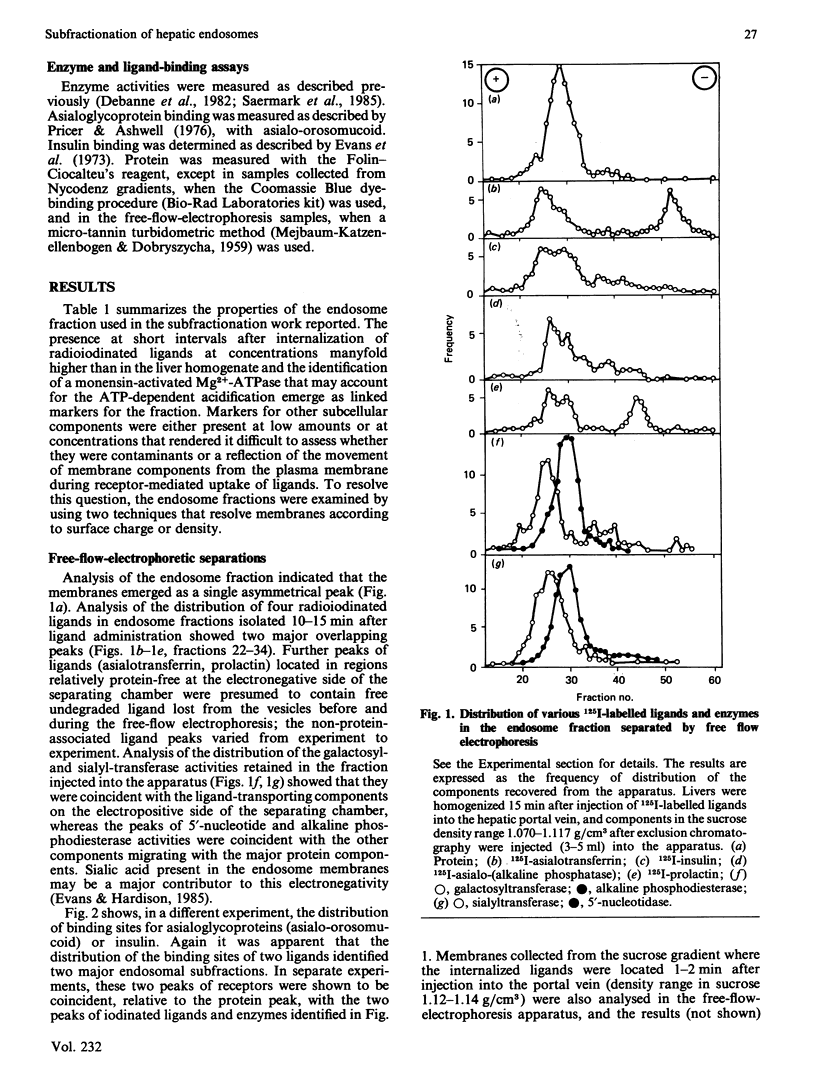
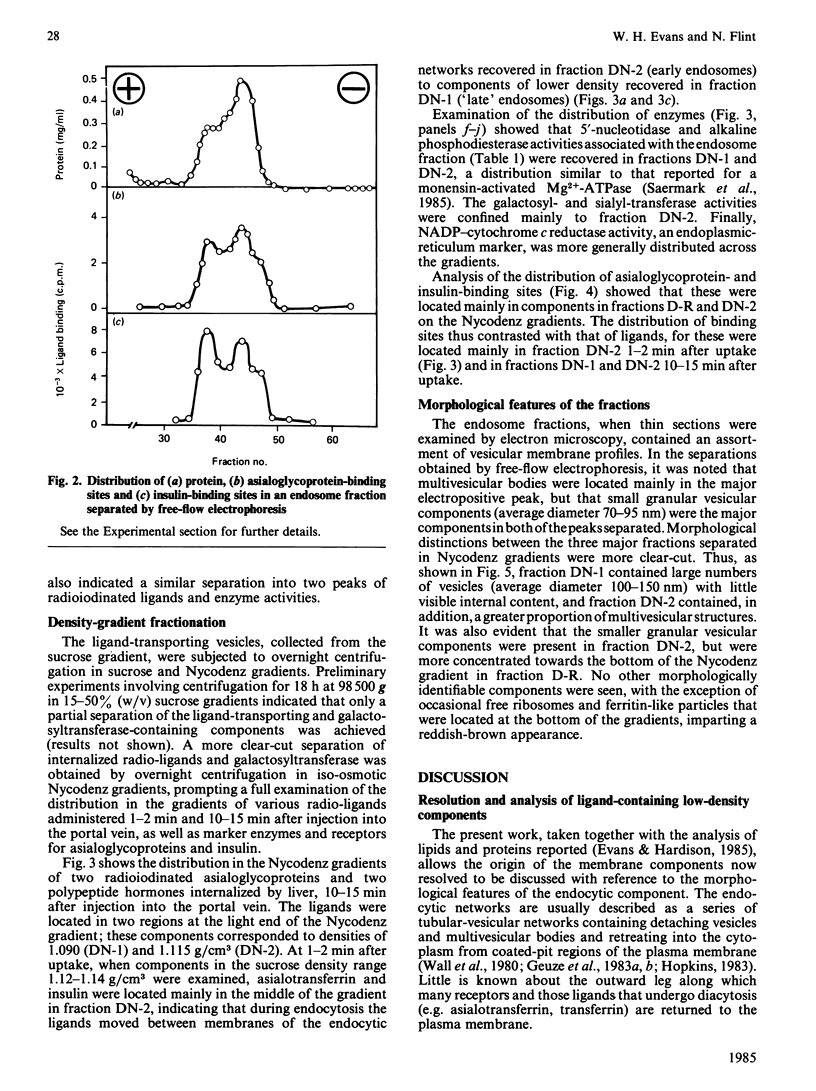
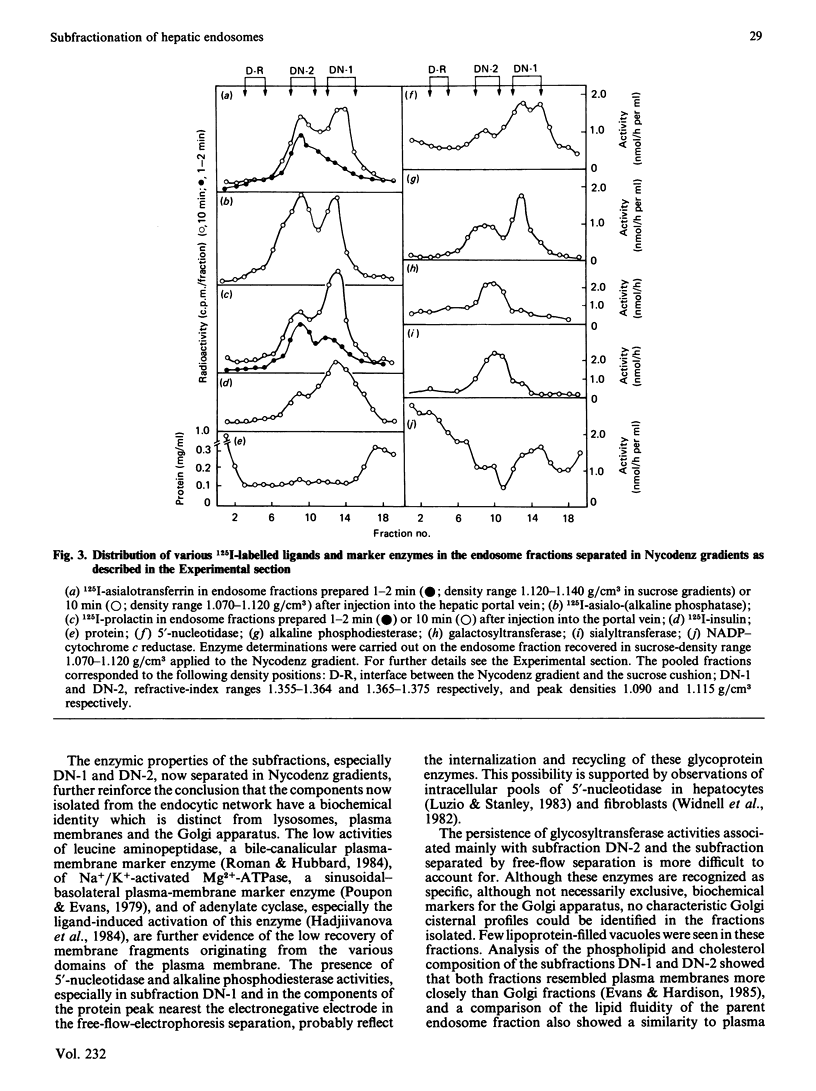
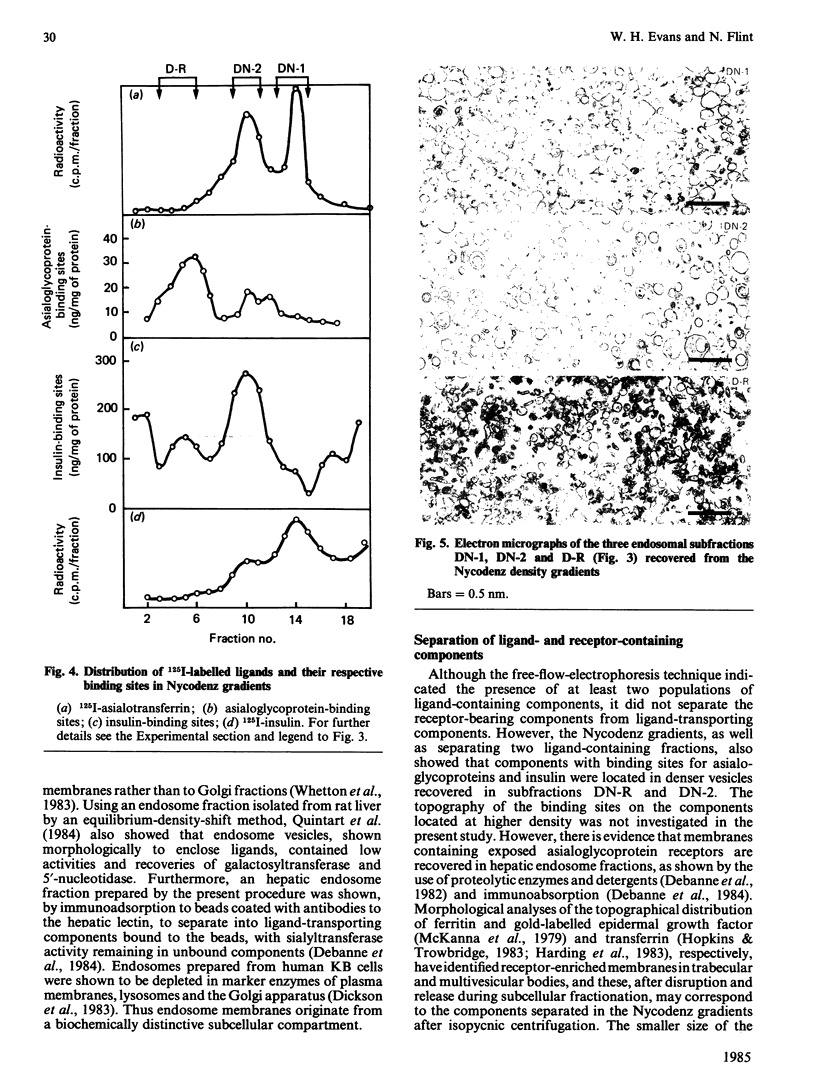
The complexity of rat liver endosome fractions containing internalized radioiodinated asialotransferrin, asialo-(alkaline phosphatase), insulin and prolactin was investigated by using free-flow electrophoresis and isopycnic centrifugation in Nycodenz gradients. Two subfractions were separated by free-flow electrophoresis. Both subfractions contained receptors for asialoglycoprotein and insulin. Glycosyltransferase activities were associated with the more electronegative vesicles, whereas 5'-nucleotidase and alkaline phosphodiesterase activities were associated with the less electronegative vesicles. Three subfractions were separated on Nycodenz gradients. Two subfractions, previously shown to become acidified in vitro, contained the ligands. At short intervals after uptake (1-2 min), ligands were mainly in subfraction DN-2 (density 1.115 g/cm3), but movement into subfraction DN-1 (density 1.090 g/cm3) had occurred 10-15 min after internalization. Low amounts of glycosyltransferase activities were associated with subfraction DN-2, and 5'-nucleotidase and alkaline phosphodiesterase activities were mainly located in subfraction DN-1. The binding sites for asialoglycoproteins and insulin were distributed towards the higher density range in the Nycodenz gradients, thus indicating a segregation of receptor-enriched vesicles and those vesicles containing the various ligands 10-15 min after internalization. Electron microscopy of the subfractions separated on Nycodenz gradients indicated that whereas the ligand-transporting fractions consisted mainly of empty vesicles (average diameter 100-150 nm), the receptor-enriched component was more granular and smaller (average diameter 70-95 nm). The properties of the endosome subfraction are used to assign their origin to the regions of the endocytic compartment where ligand-receptor dissociation and separation occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- DeBanne M. T., Bolyos M., Gauldie J., Regoeczi E. Two populations of prelysosomal structures transporting asialoglycoproteins in rat liver. Proc Natl Acad Sci U S A. 1984 May;81(10):2995–2999. doi: 10.1073/pnas.81.10.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne M. T., Evans W. H., Flint N., Regoeczi E. Receptor-rich intracellular membrane vesicles transporting asialotransferrin and insulin in liver. Nature. 1982 Jul 22;298(5872):398–400. doi: 10.1038/298398a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Bergeron J. J., Geschwind I. I. Distribution of insulin receptor sites among liver plasma membrane subfractions. FEBS Lett. 1973 Aug 15;34(2):259–262. doi: 10.1016/0014-5793(73)80807-5. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Bruning J. W. Studies on the distribution of some histocompatibility antigens in mouse liver plasma membrane and microsomal fractions. Immunology. 1970 Nov;19(5):735–741. [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hardison W. G. Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem J. 1985 Nov 15;232(1):33–36. doi: 10.1042/bj2320033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Schwartz A. L. The pathway of the asialoglycoprotein-ligand during receptor-mediated endocytosis: a morphological study with colloidal gold/ligand in the human hepatoma cell line, Hep G2. Eur J Cell Biol. 1983 Nov;32(1):38–44. [PubMed] [Google Scholar]
- Hadjiivanova N., Flint N., Evans W. H., Dix C., Cooke B. A. Endocytosis of beta-adrenergic ligands by rat liver. Comparison of beta-adrenergic receptor and adenylate cyclase distribution in endosome and plasma-membrane fractions. Biochem J. 1984 Sep 15;222(3):749–754. doi: 10.1042/bj2220749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindberg G. M., Ford T., Blomhoff R., Rickwood D., Berg T. Separation of endocytic vesicles in Nycodenz gradients. Anal Biochem. 1984 Nov 1;142(2):455–462. doi: 10.1016/0003-2697(84)90489-5. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Stanley K. K. The isolation of endosome-derived vesicles from rat hepatocytes. Biochem J. 1983 Oct 15;216(1):27–36. doi: 10.1042/bj2160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon R. E., Evans W. H. Biochemical evidence that Na+,K+-ATPase is located at the lateral region of the hepatocyte surface membrane. FEBS Lett. 1979 Dec 15;108(2):374–378. doi: 10.1016/0014-5793(79)80567-0. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. Subcellular distribution of a mammalian hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Dec 10;251(23):7539–7544. [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in rat liver: purification and enzymic characterization of low density organelles involved in uptake of galactose-exposing proteins. J Cell Biol. 1984 Mar;98(3):877–884. doi: 10.1083/jcb.98.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoeczi E., Chindemi P. A., Debanne M. T., Hatton M. W. Dual nature of the hepatic lectin pathway for human asialotransferrin type 3 in the rat. J Biol Chem. 1982 May 25;257(10):5431–5436. [PubMed] [Google Scholar]
- Regoeczi E., Taylor P., Debanne M. T., März L., Hatton M. W. Three types of human asialo-transferrin and their interactions with the rat liver. Biochem J. 1979 Nov 15;184(2):399–407. doi: 10.1042/bj1840399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman L. M., Hubbard A. L. A domain-specific marker for the hepatocyte plasma membrane. II. Ultrastructural localization of leucine aminopeptidase to the bile canalicular domain of isolated rat liver plasma membranes. J Cell Biol. 1984 Apr;98(4):1488–1496. doi: 10.1083/jcb.98.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saermark T., Flint N., Evans W. H. Hepatic endosome fractions contain an ATP-driven proton pump. Biochem J. 1985 Jan 1;225(1):51–58. doi: 10.1042/bj2250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens H. B., Hardonk M. J., Meijer D. K. A kinetic study of hepatic uptake of canine intestinal alkaline phosphatase in the rat. Liver. 1982 Mar;2(1):1–13. doi: 10.1111/j.1600-0676.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem. 1984;16(3):207–233. doi: 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Houslay M. D., Dodd N. J., Evans W. H. The lipid fluidity of rat liver membrane subfractions. Biochem J. 1983 Sep 15;214(3):851–854. doi: 10.1042/bj2140851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Schneider Y. J., Pierre B., Baudhuin P., Trouet A. Evidence for a continual exchange of 5'-nucleotidase between the cell surface and cytoplasmic membranes in cultured rat fibroblasts. Cell. 1982 Jan;28(1):61–70. doi: 10.1016/0092-8674(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J Cell Biochem. 1984;26(4):231–246. doi: 10.1002/jcb.240260404. [DOI] [PubMed] [Google Scholar]



