Abstract
Cellular senescence, characterized by cell cycle arrest, can result in tissue dysfunction when senescent cells persist and accumulate. Periodontitis, a chronic inflammatory condition caused by the interaction between bacteria and the immune system of the host, primarily manifests as damage to periodontal tissues. Aging and inflammation are interlinked processes that exacerbate each other. The progression of localized chronic periodontal inflammation is often accelerated in conjunction with tissue and organ aging. The presence of senescent cells and release of inflammatory cytokines, immune modulators, growth factors and proteases that are associated with the senescence-associated secretory phenotype contribute to the deterioration of periodontal tissues. The present review aimed to elucidate the mechanisms of cellular senescence and its potential impact on periodontitis, offering novel insights for modulating the inflammatory microenvironment of periodontal tissues.
Keywords: cellular senescence, periodontitis, senescence-associated secretory phenotype
1. Introduction
Aging encompasses a gradual decline in physiological and pathological functions. Physiological aging involves the natural deterioration of organs and systems over time, while pathological aging is influenced by diseases, environmental factors or other abnormal causes, including chronic disease, inflammation and genetic mutation (1). There is not an absolute boundary between physiological and pathological aging and both can influence the onset of cellular senescence (2,3). Cellular senescence is the irreversible halting of cell division, which is induced by external pro-senescence factors. IGFBP5 actively contributes to promoting senescence and can induce senescence in neighboring cells. The senescence-associated secretory phenotype (SASP) contributes to this process through the secretion of interleukins (ILs), tumor necrosis factor (TNF)-α, prostaglandin E2, interferon (INF)-β and IFN-γ. Periodontitis, a chronic inflammatory disease, disproportionately affects the patients aged 60–65 years, with aging being a notable risk factor that exacerbates alveolar bone and tooth loss (4). In developing countries, periodontal disease is common in the elderly, with 62–97.00% having mild periodontitis and 20.00 to 48.00% having severe periodontitis (5). Various stimuli such as oxidative stress, proinflammatory factors, microbial infections or activation of signaling pathways can induce senescence in cells that is independent of telomere shortening. Due to cell cycle arrest, senescent cells exhibit altered expression profiles of proteins and transcription factors that are associated with the regulation of the cell cycle (6). While bacteria may induce inflammation, the immune response of the host is the primary driver of periodontal tissue destruction. Cellular senescence can compromise bacterial clearance and immune defenses (7). Targeting cellular senescence presents a novel approach to understanding the mechanisms and clinical management of periodontitis. The present review investigated the mechanisms of cellular senescence and the characterization of periodontal tissues during cellular senescence (Fig. 1).
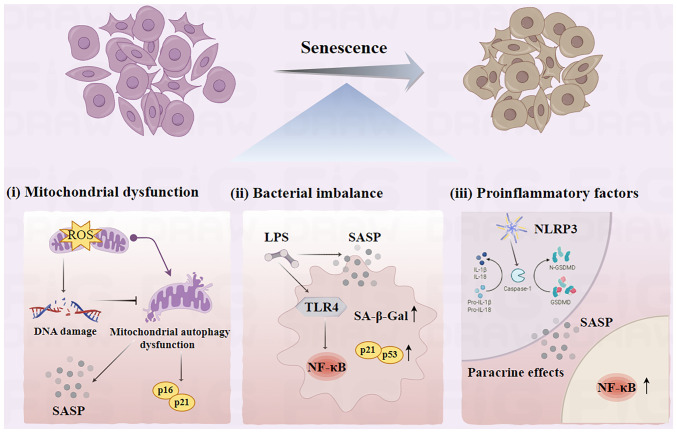
Figure 1.
Mechanisms of cellular senescence. (i) Impaired scavenging of reactive oxygen radicals causes DNA damage. This leads to an accumulation of senescence-associated lipofuscin, and inhibits mitochondrial autophagy, which increases protein expression of the cellular senescence markers p16 and p21. (ii) Bacterial flora imbalance can lead to an increase in the secretion of SASP-associated proteins and an activation of the downstream NF-κB pathway via TLR4. This promotes the release of inflammatory cytokines, which in turn promotes cell injury. (iii) NLRP3 recognizes caspase-1, which converts IL-1β and IL-18 precursors to mature IL-1β and IL-18, triggering pyroptosis. Senescent cells secrete SASP-associated protein (such as IL-1α, IL-1β, IL-6 and IL-8), which affect the functions of neighboring cells through paracrine and autocrine signaling mechanisms. SASP, senescence-associated secretory phenotype; TLR4, Toll-like receptor 4; NLRP3, NOD-like receptor pyrin domain-containing protein 3; Caspase-1, cysteinyl aspartate specific proteinases-1; IL, interleukins; ROS, reactive oxygen species; LPS, lipopolysaccharides; SA-β-Gal, senescence-associated β-galactosidase; GSDMD, gasdermin D.
2. Mechanisms of cellular senescence
Oxidative stress
Aging is characterized by progressive degeneration, which is influenced by alterations in the oxidation-reduction status and inflammatory responses triggered by oxidative stress (8). This results in an increased production of reactive oxygen species (ROS) within the body, leading to abnormal levels of oxidative stress. Specific increases in the levels of ROS are hypothesized to serve an important role in initiating and perpetuating the cellular aging processes. The accumulation of ROS can result in DNA damage and subsequent cell cycle arrest (9). DNA damage can further lead to telomere shortening, DNA methylation, histone deacetylation and mitochondrial dysfunction, thereby instigating transcriptome alterations that are associated with aging (10,11).
Mitochondrial dysfunction is a hallmark of aging (12). Mitochondrial autophagy, vital for maintaining mitochondrial quality control, eliminates dysfunctional mitochondria to maintain cellular homeostasis. It can modulate the cellular senescence phenotype by suppressing transcription of SASP-associated genes (13). The accumulation of lipofuscin is a distinctive feature of senescent cells, and oxidative stress-induced DNA damage during senescence promotes its accumulation (14). Moreover, it is associated with impaired mitochondrial autophagy function, exhibiting a positive association with the expression levels of cell aging markers p16 and p21 (11,15,16). Oxidative stress-induced senescence (SIPS) is implicated in the pathogenesis of age-associated macular degeneration and is known to increase secretion of SASP-associated proteins (15,16). In periodontal tissue, increases in the SIPS levels have detrimental effects that are comparable to inflammatory factors and promote the secretion of SASP-associated proteins (17). Targeting mitochondrial autophagy pathways in mesenchymal stem cells in various degenerative conditions, such as intervertebral disc degeneration (IVDD) and osteoarthritis (OA), may enhance cartilage repair and regeneration capabilities (18–20).
Bacterial imbalance
Aging is associated with alterations in the composition of microbial communities. For example, in the gut, the microbiota can influence the aging of gut cells by regulating the intestinal epithelial barrier, gut immune system and gut metabolism. In the skin, the microbiota can influence skin cell senescence by regulating the integrity of the stratum corneum, the skin immune system and skin metabolism. Previous studies reveal a shift in the microbiome with age, demonstrating a positive association between aging and the presence of gram-negative bacteria such as Porphyromonas gingivalis (21,22). Furthermore, aging is characterized by an increased responsiveness of periodontal cells to the oral microbiota and mechanical stress (23). A previous study indicates that bacterial lipopolysaccharides (LPS) can induce cellular senescence and increase expression levels of SASP-associated genes (24). Prolonged exposure to P. gingivalis LPS can prompt periodontal cell senescence due to the activation of the cell cycle arrester molecule p53 (25). LPS also mediate microglia activation and ischemic brain injury via the NF-κB signaling pathway (26). Additionally, LPS from Escherichia coli is indicated to increase the activity of senescence-associated β-galactosidase, increase the expression levels of cell cycle inhibitor proteins p21 and p53, and impede human pulp stem cell growth (25,27,28). Increased expression of LPS by P. gingivalis may stimulate NF-κB activation in periodontal ligament stem cells (PDLSCs), exacerbate the high glucose microenvironment and increase the expression of proinflammatory cytokines (namely, ILs, TNF and INF) and matrix metalloproteinases (MMPs) such as MMP-1 (29,30). LPS can also trigger the downstream NF-κB pathway via Toll-like receptor 4, prompting the release of inflammatory cytokines such as TNF-α, IL-6 and IL-1β that contribute to cellular damage. Activation of NF-κB also results in an increased expression of p53 and p21 in the PDLSC nucleus, which is pivotal in promoting cellular aging (31,32).
Proinflammatory factors
A potential feedback loop exists between inflammatory cytokines and cellular senescence, which could hasten the progression of the senescence of inflammatory cell (33). Senescent cells are more vulnerable to harm from external stimuli, including proinflammatory factors and bacterial virulence factors. The NOD-like receptor pyrin domain-containing protein 3 inflammasome recognizes pathogen-associated molecular patterns or damage-associated molecular patterns, activating cysteinyl aspartate specific proteinases-1 (Caspase-1) (34). Caspase-1 cleaves the protein Gasdermin D, which then converts the precursor forms of IL-1β and IL-18 into mature forms, thereby initiating pyroptosis (35,36). IL-1β can promote paracrine cellular senescence and NF-κB activation, activating a cascade of an inflammatory-induced senescence (37). The accumulation of pyroptotic macrophages (MΦs) in gingival tissue under high-glucose conditions can stimulate IL-1β secretion and paracrine senescence in neighboring cells (38). Furthermore, proinflammatory cytokines (such as ILs, TNF and INF) generate the ROS themselves and then the ROS induce epithelial cell senescence, the ROS activate the Eotaxin-1/CCL11 pathway leading to fibroblast senescence (39,40).
Senescent cells, with a SASP, release various proinflammatory factors (such as IL-1α, IL-1β, IL-6 and IL-8), growth factors (such as hepatocyte growth factor, TGF-β and granulocyte macrophage colony-stimulating factor), chemokines [such as chemokine (C-X-C motif) ligand (CXCL)-1/3 and CXCL-10] and MMP-8 and MMP-9. The SASP can promote chronic inflammation through paracrine effects, affecting neighboring stem cells, fibroblasts, immune cells, epithelial cells and endothelial cells via paracrine and autocrine signaling mechanisms (41). In the aging heart, MMP-9 activates the transition of the MΦ phenotype to the proinflammatory M1 subtype can induce inflammation due to its increased secretion of TNF-α, IL-1β, IL-6 and other inflammatory factors (42). Activation of the SASP initiates a milieu of chronic inflammation in addition to the age-induced stressors, leading to a self-perpetuating cycle of senescent cell accumulation (43).
3. Senescence-associated alterations in the periodontal microenvironment
Alveolar bone remodeling ability and cellular senescence
PDLSCs possess self-renewal and multi-directional differentiation capabilities, which are important for periodontal tissue regeneration and osteogenic differentiation (44). The senescence of PDLSCs reduces their osteogenic potential, leading to periodontal tissue destruction via inflammation and the induction of the SASP (Fig. 2). This process reduces the regenerative capacity of periodontal tissues in periodontitis. In patients with diabetes, oxidative stress induces telomere dysfunction and PDLSC senescence, reducing periodontal bone tissue regeneration and increasing bone loss in periodontitis (45–47).
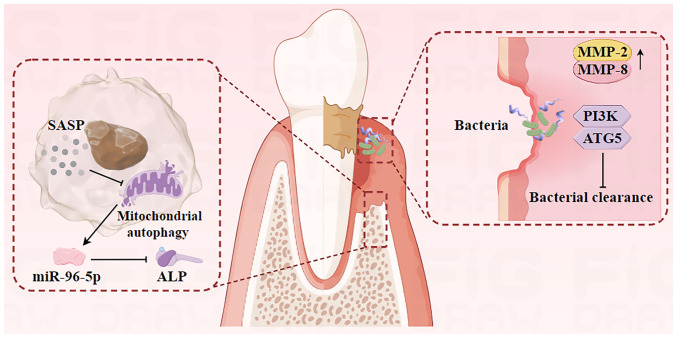
Figure 2.
Cellular senescence affects alveolar bone metabolism through secretion of SASP-associated proteins. MiR-96-5p promotes osteoblast senescence, leading to decreased bone differentiation. Senescence-associated dysbiosis compromises the integrity of the epithelial barrier and further destroys deeper tissues. Decreased levels of developmental endothelial locus-1, PI3K and ATG5 affect periodontal bacterial clearance, thus exacerbate periodontal inflammation. SASP, senescence-associated secretory phenotype; miR-96-5p, micro-RNA-96-5p; ALP, alkaline phosphatase; MMP, matrix metalloproteinases; ATG5, autophagy-related protein 5.
Aging impacts the proliferation and differentiation of bone marrow mesenchymal stem cells (BMSCs), with senescent cells involved in senile osteoporosis-related bone loss (48). Sirtuin (SIRT), a NAD-dependent deacetylase family member, is a potential therapeutic target for age-related diseases (49). SIRT1, an upstream regulator of mitochondrial autophagy, can inhibit age-related degenerative changes in IVDD and OA (18,50). The SIRT1/PTEN-induced kinase 1/Parkin pathway-mediated mitophagy activation reduces renal tubular epithelial cell senescence (51). The balance between osteoblast and osteoclast activity maintains bone mass, and osteoblasts are important in bone formation through matrix synthesis, substance secretion and tissue mineralization. Upregulation of SIRT1 expression levels activates the PI3K/Akt/mTOR pathway to promote mitophagy, enhancing osteoblast proliferation and viability (52).
Inhibition of micro (mi)RNA-96-5p expression levels in osteoblasts markedly downregulates the mRNA of alkaline phosphatase (the bone differentiation factor), which indicates that miRNA-96-5p promotes osteoblast senescence, leading to decreased bone differentiation. miRNA-96-5p is likely crucial in regulating osteoblast degenerative changes during aging (53,54). The accumulation of senescent cells in the alveolar bone can stimulate the secretion of SASP-associated protein, which exerts a potent paracrine effect on osteoblasts, inhibiting their function and reducing bone regeneration in periodontitis (23,55). Inflammatory bone loss in periodontitis, characterized by osteoclast activity, is exacerbated by aging, which enhances osteoclast production (56). Osteoblast-secreted osteoprotegerin (OPG) mitigates bone loss by inhibiting the receptor activator of NF-κB (RANK) ligand/RANK pathway. However, the reduction in osteoblast numbers with aging reduces OPG levels, contributing to osteoclast-induced bone loss (57,58). These findings underscore the causal role of senescent cells and their SASPs in alveolar bone loss and suggest that targeting senescent cells could increase the alveolar bone remodeling capabilities.
Bacterial clearance capacity and cellular senescence
Gingival fibroblasts (GFs) are predominant cells within the gingival connective tissue and serve an important role in regulating periodontal inflammation. The proliferative capacity of GFs and the mitotic activity of PDLSCs may reduce with age. This reduction is associated with increased mRNA levels of MMP-2 and MMP-8, leading to the increased degradation of the extracellular matrix (59). Chronic bacterial assaults can induce a reduction in the amount of epithelial growth, compromising the integrity of the epithelial barrier (60). Once this barrier is breached, bacteria and their virulence factors such as endotoxin and exotoxin, can penetrate deeper connective tissues, exacerbating periodontal tissue damage (61).
Neutrophils are important for pathogen clearance in the immune response to periodontitis. Age-related neutrophil dysfunction may disrupt the neutrophil homeostasis in periodontal tissues, impairing bacterial clearance and resulting in subsequent damage to periodontal tissues (62). Previous studies demonstrate developmental endothelial locus-1 mRNA and protein expression levels are downregulated in the periodontal tissue of aged mice compared with that of young mice (63,64). This downregulation leads to an excessive recruitment of neutrophils and inflammatory bone resorption. Furthermore, in elderly hosts aged >65 years, excessive or dysregulated PI3K activity impairs the accuracy of neutrophil migration (65,66). Aging mice have a deficiency in autophagy-related protein 5, resulting in decreased autophagy levels and impaired release of neutrophil extracellular traps (NETs) (67–69). This impairment hinders the clearance of pathogenic bacteria and their metabolites in the gingival sulcus by NETs. In summary, cellular senescence reduces the clearance of existing periodontal bacteria, compromising the integrity of the epithelial barrier and exacerbating local periodontal inflammation.
Organizational defense effectiveness and cellular senescence
MΦs serve an important role in the innate immunity as the initial defense against pathogenic microorganisms. Monitoring periodontitis activity may rely on the phenotypic polarization of MΦs in order for them to modulate the immune response to the subgingival biofilm and mitigate alveolar bone loss (70). Aging-related M1 to M2 repolarization failure can lead to an increase in osteoclast activation, a reduction in osteoblast formation, an increase in bone resorption and a decrease in bone formation. Senescent MΦs are implicated in perpetuating chronic inflammation during bone remodeling, as well as inducing a senescent state in young BMSCs and diminishing their osteogenic potential (71). MΦ M1 polarization is associated with PDLSC senescence in diabetic periodontitis microenvironments. By contrast, M2 polarization potentially increases the expression levels of osteogenesis-related cytokines, such as runt-related transcription factor 2, alkaline phosphatase and osteocalcin, exerts anti-aging effects and facilitates the osteogenic differentiation of PDLSCs (72,73). Additionally, the paracrine factors released by PDLSCs can stimulate the expression of CD163, a surface marker associated with M2-MΦs, which contributes to macrophage M2 polarization (74).
Dendritic cells (DCs) represent a diverse cell population and inhibiting their function results in reduced responses of the adaptive immune system as well as an increased susceptibility to periodontal disease. A previous study indicates that the quantity of DCs in the peripheral blood fluctuates with age, potentially contributing to the increased vulnerability of older patients to infection compared with younger patients (75). Furthermore, alterations in the functionality of DCs contribute to immune dysregulation and the onset of chronic inflammation. In vitro, infection of DCs by the non-living oral pathogen P. gingivalis triggers activation of the SASP and subsequent bone loss (76).
Langerhans cells (LCs), a subset of DCs located in the oral mucosal epithelium, likely serve a role in initiating and perpetuating periodontal diseases (77). A decrease in the number of epithelial LCs and their dendritic structures in elderly individuals aged >75 years with periodontitis, which are distinct compared with those in adults, may compromise their removal of pathogenic bacteria (78,79). Reducing LCs in the aging epidermis impairs the immunoregulatory functions of the skin and compromises barrier integrity, antimicrobial defenses and overall cell protection capabilities (80,81). Consequently, immune cell senescence may disrupt the homeostasis of periodontal defense mechanisms.
4. Conclusions and prospects
In conclusion, the prevalence and severity of periodontitis are associated with cellular senescence. The intricate changes in cellular senescence can impair the effective removal of pathogens by immune cells. The interaction of intracellular and extracellular aging environments can lead to the deterioration of cells and factors involved in bone metabolism, tissue defense and immune response in periodontal tissues, which exacerbates the progression of periodontitis. Furthermore, a comprehensive literature review underscores the complexity of the association between cellular senescence and inflammation. The precise nature of the association between cellular senescence and inflammation is yet to be completely understood, with the possibility of a causal or bidirectional association.
The current understanding suggests that the cascade of changes triggered by cellular senescence serves a role in the development of periodontal disease, potentially explaining the increased prevalence of this condition among the elderly aged >65 years. Increasing the understanding of the association between cellular senescence and periodontitis could offer a novel approach to preventing and treating periodontal diseases in older individuals. Further investigation is required to assess the applicability of these mechanisms to other age-related diseases such as hypertension, diabetes in clinical settings.
Acknowledgements
Not applicable.
Funding Statement
The present study was funded by the National Natural Science Foundation of China (grant no. 82201080), the High-level Talents Project of Hainan Natural Science Foundation (grant no. 821RC687) and the Innovative Scientific Research Project for Postgraduates of Hainan Medical College (grant no. Qhys2022-280).
Availability of data and materials
Not applicable.
Authors' contributions
ZG conceived and designed the present review. XL wrote most of the manuscript. DS, JZ, YD and QY edited the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lu Y, Tang X, Wang W, Yang J, Wang S. The role of deacetylase SIRT1 in allergic diseases. Front Immunol. 2024;15:1422541. doi: 10.3389/fimmu.2024.1422541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jothi D, Kulka LAM. Strategies for modeling aging and age-related diseases. NPJ Aging. 2024;10:32. doi: 10.1038/s41514-024-00161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Zhou D, Liu O, Chen H, Wang Y, Zhou Y. Cellular senescence and periodontitis: Mechanisms and therapeutics. Biology (Basel) 2022;11:1419. doi: 10.3390/biology11101419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma SK, Singh N, Jha AK, Tigga C, Noorani MK, Ekram S, Gupta V. Prevalence of periodontal disease among patients reporting to tertiary care hospital in Ranchi. J Pharm Bioallied Sci. 2024;16((Suppl 1)):S838–S840. doi: 10.4103/jpbs.jpbs_1051_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan J, Chen S, Yi Z, Zhao R, Zhu J, Ding S, Wu J. The role of p21 in cellular senescence and aging-related diseases. Mol Cells. 2024;18:100113. doi: 10.1016/j.mocell.2024.100113. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Luo Y, Yuan Z, Tian Y, Jin T, Xu F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol Cancer. 2024;23:181. doi: 10.1186/s12943-024-02096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8:239. doi: 10.1038/s41392-023-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusciano D, Bagnoli P. Oxygen, the Paradox of Life and the Eye. Front Biosci (Landmark Ed) 2024;29:319. doi: 10.31083/j.fbl2909319. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li Y, Peng Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97:1439–1451. doi: 10.1007/s00204-023-03476-6. [DOI] [PubMed] [Google Scholar]
- 11.Terao R, Ahmed T, Suzumura A, Terasaki H. Oxidative stress-induced cellular senescence in aging retina and age-related macular degeneration. Antioxidants (Basel) 2022;11:2189. doi: 10.3390/antiox11112189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen J, Pan T, Li H, Fan H, Liu J, Cai Z, Zhao B. Role of mitophagy in the hallmarks of aging. J Biomed Res. 2022;37:1–14. doi: 10.1155/2022/7800298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Xu L, Li Y, Jia S, Wang G, Cen X, Xu Y, Cao Z, Wang J, Shen N, et al. Mitophagy defect mediates the aging-associated hallmarks in Hutchinson-Gilford progeria syndrome. Aging Cell. 2024;23:e14143. doi: 10.1111/acel.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilie OD, Ciobica A, Riga S, Dhunna N, McKenna J, Mavroudis I, Doroftei B, Ciobanu AM, Riga D. Mini-Review on lipofuscin and aging: focusing on the molecular interface, the biological recycling mechanism, oxidative stress, and the gut-brain axis functionality. Medicina (Kaunas) 2020;56:626. doi: 10.3390/medicina56110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KS, Lin S, Copland DA, Dick AD, Liu J. Cellular senescence in the aging retina and developments of chemotherapies for age-related macular degeneration. J Neuroinflammation. 2021;18:32. doi: 10.1186/s12974-021-02088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasiak J. Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci. 2020;77:789–805. doi: 10.1007/s00018-019-03420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M, Li J, Song J. Metformin prevents oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology. 2020;21:13–27. doi: 10.1007/s10522-019-09838-x. [DOI] [PubMed] [Google Scholar]
- 18.Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17:2082–2092. doi: 10.1080/15548627.2020.1822097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Song X. The relationship between Alzheimer's disease and thyroiditis: A two-sample Mendelian randomization study. Medicine (Baltimore) 2023;102:e35712. doi: 10.1097/MD.0000000000035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Sun B, Wang H, Cai Y, Chu D, Cao R, Wang Z. Autophagy regulates age-related delayed jawbone regeneration and decreased osteoblast osteogenesis by degrading FABP3. FASEB J. 2024;38:e23824. doi: 10.1096/fj.202400549RR. [DOI] [PubMed] [Google Scholar]
- 21.Ebersole JL, Nagarajan R, Kirakodu S, Gonzalez OA. Oral microbiome and gingival gene expression of inflammatory biomolecules with aging and periodontitis. Front Oral Health. 2021;2:725115. doi: 10.3389/froh.2021.725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz JL, Peña N, Kawar N, Zhang A, Callahan N, Robles SJ, Griebel A, Adami GR. Old age and other factors associated with salivary microbiome variation. BMC Oral Health. 2021;21:490. doi: 10.1186/s12903-021-01828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Chung J. Aging aggravates periodontal inflammatory responses and alveolar bone resorption by porphyromonas gingivalis infection. Curr Issues Mol Biol. 2023;45:6593–6604. doi: 10.3390/cimb45080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki K, Susaki EA, Nagaoka I. Lipopolysaccharides and cellular senescence: Involvement in Atherosclerosis. Int J Mol Sci. 2022;23:11148. doi: 10.3390/ijms231911148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020;132:115220. doi: 10.1016/j.bone.2019.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung YS, Park JH, Kim H, Kim SY, Hwang JY, Hong KW, Bae SS, Choi BT, Lee SW, Shin HK. Probucol inhibits LPS-induced microglia activation and ameliorates brain ischemic injury in normal and hyperlipidemic mice. Acta Pharmacol Sin. 2016;37:1031–1044. doi: 10.1038/aps.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng G, Zheng K, Cao T, Zhang J, Lian M, Huang D, Wei C, Gu Z, Feng X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology. 2018;70:1023–1035. doi: 10.1007/s10616-017-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao H, Nepovimova E, Heger Z, Valko M, Wu Q, Kuca K, Adam V. Role of hypoxia in cellular senescence. Pharmacol Res. 2023;194:106841. doi: 10.1016/j.phrs.2023.106841. [DOI] [PubMed] [Google Scholar]
- 29.Nareika A, Im YB, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose enhances lipopolysaccharide-stimulated CD14 expression in U937 mononuclear cells by increasing nuclear factor kappaB and AP-1 activities. J Endocrinol. 2008;196:45–55. doi: 10.1677/JOE-07-0145. [DOI] [PubMed] [Google Scholar]
- 30.Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, rede S. Hypoxia and P. gingivalis synergistically induce HIF-1 and NF-κB activation in PDL cells and periodontal diseases. Mediators Inflamm. 2015;2015:438085. doi: 10.1155/2015/438085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P, Moro S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16:148. doi: 10.1186/s12974-019-1538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin K, Patten D, Gough S, de Barros Gonçalves S, Chan A, Olan I, Cassidy L, Poblocka M, Zhu H, Lun A, et al. Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance. Genes Dev. 2022;36:533–549. doi: 10.1101/gad.349585.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Yin H, Yuan H, Wang E, Wang C, Li H, Geng X, Zhang Y, Bai J. IL-10 deficiency aggravates cell senescence and accelerates BLM-induced pulmonary fibrosis in aged mice via PTEN/AKT/ERK pathway. BMC Pulm Med. 2024;24:443. doi: 10.1186/s12890-024-03260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang H, Du Y, Tan Z, Li D, Xie J. METTL14-mediated HOXA5 m6A modification alleviates osteoporosis via promoting WNK1 transcription to suppress NLRP3-dependent macrophage pyroptosis. J Orthop Translat. 2024;48:190–203. doi: 10.1016/j.jot.2024.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S, Sun C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63 doi: 10.1111/jpi.12414. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Li X, Wang Y, Wei Y, Wei X. Involvement of inflammasomes in tumor microenvironment and tumor therapies. J Hematol Oncol. 2023;16:24. doi: 10.1186/s13045-023-01407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-Zamudio RI, Robinson L, Roux PF, Bischof O. SnapShot: Cellular senescence pathways. Cell. 2017;170:816–816.e1. doi: 10.1016/j.cell.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Zhao P, Yue Z, Nie L, Zhao Z, Wang Q, Chen J, Wang Q. Hyperglycemia-associated macrophage pyroptosis accelerates periodontal inflame-aging. J Clin Periodontol. 2021;48:1379–1392. doi: 10.1111/jcpe.13517. [DOI] [PubMed] [Google Scholar]
- 39.Tsirpanlis G. Cellular senescence and inflammation: A noteworthy link. Blood Purif. 2009;28:12–14. doi: 10.1159/000210032. [DOI] [PubMed] [Google Scholar]
- 40.Lavandoski P, Pierdoná V, Maurmann RM, Grun LK, Guma F, Guma FTCR, Barbé-Tuana FM. Eotaxin-1/CCL11 promotes cellular senescence in human-derived fibroblasts through pro-oxidant and proinflammatory pathways. Front Immunol. 2023;14:1243537. doi: 10.3389/fimmu.2023.1243537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue Z, Nie L, Zhao P, Ji N, Liao G, Wang Q. Senescence-associated secretory phenotype and its impact on oral immune homeostasis. Front Immunol. 2022;13:1019313. doi: 10.3389/fimmu.2022.1019313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2008;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for targeting senescent cells in human disease. Nat Aging. 2021;1:870–879. doi: 10.1038/s43587-021-00121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials. 2012;33:6974–6986. doi: 10.1016/j.biomaterials.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Zhang B, Wang H, Zhao X, Zhang Z, Ding G, Wei F. The effect of aging on the biological and immunological characteristics of periodontal ligament stem cells. Stem Cell Res Ther. 2020;11:326. doi: 10.1186/s13287-020-01846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang L, Li T, Chang Y, Wang Z, Li Y, Wang F, Sui L. Diabetic oxidative stress-induced telomere damage aggravates periodontal bone loss in periodontitis. Biochem Biophys Res Commun. 2022;614:22–28. doi: 10.1016/j.bbrc.2022.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Du TT, Liu N, Zhang W, Shi HG, Zhang T. Effect of aging on proliferative and differentiation capacity of human periodontal ligament stem cells. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:360–366. doi: 10.3969/j.issn.1673-4254.2017.03.14. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Föger-Samwald U, Kerschan-Schindl K, Butylina M, Pietschmann P. Age Related osteoporosis: Targeting cellular senescence. Int J Mol Sci. 2022;23:2701. doi: 10.3390/ijms23052701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, Shu G, Tian Y, Zhao X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. doi: 10.3389/fimmu.2022.943321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao H, Zhou X, Xu B, Hu H, Guo J, Wang M, Li N, Jun Z. Advances in the study of mitophagy in osteoarthritis. J Zhejiang Univ Sci B. 2024;25:197–211. doi: 10.1631/jzus.B2300402. (In English, Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T, Yang Q, Zhang X, Qin R, Shan W, Zhang H, Chen X. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 2020;257:118116. doi: 10.1016/j.lfs.2020.118116. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Jiang T, Wang Y, Guo L. The Role and Mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci Rep. 2019;9:18424. doi: 10.1038/s41598-019-44766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu BW, Guo JD, Wu MS, Liu Y, Lu M, Zhou YH, Han HW. Osteoblast-derived lipocalin-2 regulated by miRNA-96-5p/Foxo1 advances the progression of Alzheimer's disease. Epigenomics. 2020;12:1501–1513. doi: 10.2217/epi-2019-0215. [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Ji X, Ouyang Y. Unfolded protein response pathways in stroke patients: A comprehensive landscape assessed through machine learning algorithms and experimental verification. J Transl Med. 2023;21:759. doi: 10.1186/s12967-023-04567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aquino-Martinez R. The emerging role of accelerated cellular senescence in periodontitis. J Dent Res. 2023;102:854–862. doi: 10.1177/00220345231154567. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Zhang X, Cheng X, Ren T, Xu W, Li J, Wang H, Zhang J. Inflammation produced by senescent osteocytes mediates age-related bone loss. Front Immunol. 2023;14:1114006. doi: 10.3389/fimmu.2023.1114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu S, Wang S. The Role of SIRT3 in the Osteoporosis. Front Endocrinol (Lausanne) 2022;13:893678. doi: 10.3389/fendo.2022.893678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng Y, Tang X. FoxO1 as the critical target of puerarin to inhibit osteoclastogenesis and bone resorption. J Pharm Pharmacol. 2024;76:813–823. doi: 10.1093/jpp/rgae033. [DOI] [PubMed] [Google Scholar]
- 59.Ben-Eltriki M, Ahmadi AR, Nakao Y, Golla K, Lakschevitz F, Häkkinen L, Granville DJ, Kim H. Granzyme B promotes matrix metalloproteinase-1 (MMP-1) release from gingival fibroblasts in a PAR1- and Erk1/2-dependent manner: A novel role in periodontal inflammation. J Periodontal Res. 2024;59:94–103. doi: 10.1111/jre.13190. [DOI] [PubMed] [Google Scholar]
- 60.Ko YK, Hong S, Kim HM, Liu M, Moon E, Kim P, Choi Y. Characterization of junctional structures in the gingival epithelium as barriers against bacterial invasion. J Periodontal Res. 2022;57:799–810. doi: 10.1111/jre.13003. [DOI] [PubMed] [Google Scholar]
- 61.Jotwani R, Palucka AK, Al-Quotub M, Nouri-Shirazi M, Kim J, Bell D, Banchereau J, Cutler CW. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: In situ, in vivo, and in vitro studies. J Immunol. 2021;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hajishengallis G, Moutsopoulos NM, Hajishengallis E, Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. 2016;28:146–158. doi: 10.1016/j.smim.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim TS, Moutsopoulos NM. Neutrophils and neutrophil extracellular traps in oral health and disease. Exp Mol Med. 2024;56:1055–1065. doi: 10.1038/s12276-024-01219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bülow S, Ederer KU, Holzinger JM, Zeller L, Werner M, Toelge M, Pfab C, Hirsch S, Göpferich F, Hiergeist A, et al. Bactericidal/permeability-increasing protein instructs dendritic cells to elicit Th22 cell response. Cell Rep. 2024;43:113929. doi: 10.1016/j.celrep.2024.113929. [DOI] [PubMed] [Google Scholar]
- 67.Xu F, Zhang C, Zou Z, Fan EKY, Chen L, Li Y, Billiar TR, Wilson MA, Shi X, Fan J. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology. 2017;151:417–432. doi: 10.1111/imm.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim TS, Silva LM, Theofilou VI, Greenwell-Wild T, Li L, Williams DW, Ikeuchi T, Brenchley L, NIDCD/NIDCR Genomics and Computational Biology Core. Bugge TH, et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J Exp Med. 2023;220:e20221751. doi: 10.1084/jem.20221751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Si G, Si F. Mendelian Randomization validates the immune landscape mediated by aggrephagy in esophageal squamous cell carcinoma patients from the perspectives of Multi-omics. J Cancer. 2024;15:1940–1953. doi: 10.7150/jca.93376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sloniak MC, Lepique AP, Nakao LYS, Villar CC. Alterations in macrophage polarization play a key role in control and development of periodontal diseases. J Indian Soc Periodontol. 2023;27:578–582. doi: 10.4103/jisp.jisp_75_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bai L, Liu Y, Zhang X, Chen P, Hang R, Xiao Y, Wang J, Liu C. Osteoporosis remission via an anti-inflammaging effect by icariin activated autophagy. Biomaterials. 2023;297:122125. doi: 10.1016/j.biomaterials.2023.122125. [DOI] [PubMed] [Google Scholar]
- 72.Gong J, Ye C, Ran J, Xiong X, Fang X, Zhou X, Yi Y, Lu X, Wang J, Xie C, Liu J. Polydopamine-Mediated immunomodulatory patch for diabetic periodontal tissue regeneration assisted by Metformin-ZIF System. ACS Nano. 2023;17:16573–16586. doi: 10.1021/acsnano.3c02407. [DOI] [PubMed] [Google Scholar]
- 73.Liu J, Chen B, Bao J, Zhang Y, Lei L, Yan F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem Cell Res Ther. 2019;10:320. doi: 10.1186/s13287-019-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corradetti B, Taraballi F, Corbo C, Cabrera F, Pandolfi L, Minardi S, Wang X, Van Eps J, Bauza G, Weiner B, Tasciotti E. Immune tuning scaffold for the local induction of a pro-regenerative environment. Sci Rep. 2017;7:17030. doi: 10.1038/s41598-017-16895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reitsema RD, Kumawat AK, Hesselink BC, van Baarle D, van Sleen Y. Effects of ageing and frailty on circulating monocyte and dendritic cell subsets. NPJ Aging. 2024;10:17. doi: 10.1038/s41514-024-00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elsayed R, Elashiry M, Liu Y, Morandini AC, El-Awady A, Elashiry MM, Hamrick M, Cutler CW. Microbially-Induced exosomes from dendritic cells promote paracrine immune senescence: Novel mechanism of bone degenerative disease in mice. Aging Dis. 2023;14:136–151. doi: 10.14336/AD.2022.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharawi H, Heyman O, Mizraji G, Horev Y, Laviv A, Shapira L, Yona S, Hovav AH, Wilensky A. The prevalence of gingival dendritic cell subsets in periodontal patients. J Dent Res. 2021;100:1330–1336. doi: 10.1177/00220345211004864. [DOI] [PubMed] [Google Scholar]
- 78.de Vasconcelos Gurgel BC, Peixe PG, Queiroz SIML, de Almeida Freitas R, de Aquino Martins ARL, Duarte PM. Comparison of immunoexpression of dendritic cells, mast cells and blood vessels in periodontal disease between adults and elderly. Clin Oral Investig. 2023;27:6823–6833. doi: 10.1007/s00784-023-05297-4. [DOI] [PubMed] [Google Scholar]
- 79.Bodineau A, Coulomb B, Folliguet M, Igondjo-Tchen S, Godeau G, Brousse N, Séguier S. Do Langerhans cells behave similarly in elderly and younger patients with chronic periodontitis? Arch Oral Biol. 2007;52:189–194. doi: 10.1016/j.archoralbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Lee HJ, Kim TG, Kim SH, Park JY, Lee M, Lee JW, Lee SH, Lee MG. Epidermal barrier function is impaired in langerhans cell-depleted mice. J Invest Dermatol. 2019;139:1182–1185. doi: 10.1016/j.jid.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 81.Oulee A, Ma F, Teles RMB, de Andrade Silva BJ, Pellegrini M, Klechevsky E, Harman AN, Rhodes JW, Modlin RL. Identification of genes encoding antimicrobial proteins in langerhans cells. Front Immunol. 2021;12:695373. doi: 10.3389/fimmu.2021.695373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.