Abstract
Background
Bangladesh has reported > 560 H5N1 outbreaks in poultry and eight human cases since 2007. Commercial chicken farms were mostly affected. Commercial chicken farms across the country use imported vaccines against H5N1 virus; however, these vaccines did not use local circulatory isolates of H5N1virus. Vaccination may have limited effectiveness in chicken because of the mismatch in terms of subtypes and clades. To test this, we conducted a mixed-method study to assess the impact of ongoing vaccination against H5N1 virus on H5N1 viral shedding through freshly dropped feces of chickens raised in commercial farms that exclusively vaccinated or did not vaccinate their chickens.
Methods
Initially, we collected vaccination coverage data from all active farms in a subdistrict of each of eight division. In each district, 25 vaccinated and 25 non-vaccinated chicken farms were selected randomly for sample collection. All samples were tested to detect avian influenza viruses using rRT-PCR.
Results
A total of 5092 poultry farms were surveyed; among them 1284 (25%) chicken farms administered vaccine against H5N1 virus. In total 21 of 400 tested farms (5%) had chicken feces samples that tested positive for AIVs; of these three were positive for H5N1 subtype of clade 2.3.2.1. Out of three H5N1 positive farms, 1 (33%) was vaccinated and 2 (67%) were unvaccinated. The chicken farms that administered vaccine against H5N1 was found protective for the detection of H5N1 viral RNA (aOR 0.39, 95% CI: 0.32–0.48). The H5N1 isolates of clade 2.3.2.1 sequenced in this study formed a cluster with the vaccine strain A/duck/Guangdong/S1322/2010 (H5N1) [Re-6].
Conclusions
The overall low vaccination coverage with low detection of H5N1 virus in commercial chickens makes it difficult to assess the effectiveness of the vaccine in reducing H5N1 viral shedding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s42522-024-00119-3.
Keywords: HPAI, H5N1, Commercial chicken, Vaccine, Bangladesh
Introduction
Highly pathogenic avian influenza (HPAI) viruses can cause severe infections in poultry and humans [1–3]. Enhanced biosecurity measures, stamping out, and movement restrictions have been implemented conventionally to control H5N1 epidemics in poultry [4]. The HPAI H5N1 virus is endemic in several Asian countries, including China, Egypt, Indonesia, India, Hong Kong, Vietnam and Bangladesh, where vaccination programs against HPAI viruses in poultry have been implemented [5]. However, there is ongoing debate among scientists about vaccine effectiveness as HPAI outbreaks have been reported among poultry in countries that vaccinate [5–11].
In 2012, the Government of Bangladesh approved vaccines against A(H5N1) in commercial chickens, and now vaccines are available throughout the country [12]. Three different types of vaccines have been imported from Europe; RE-6 and H5N2 inactivated vaccine, which are used in commercial chickens, and the rHVT-H5 vectored vaccine, which is used in day-old-chicks [13]. The proportion of chicken farms that are being vaccinated in Bangladesh has not been reported. The lack of information on vaccination coverage indicates inadequate monitoring. Viruses from Bangladesh were not used in these imported vaccines. RE-6 inactivated vaccine contains clade 2.3.2.1b of influenza A(H5N1) viruses, A(H5N2) inactivated vaccine contains A/Potsdam/1986(H5N2) virus; and the rHVT-H5 vectored vaccine contains clade 2.2 of A(H5N1) viruses [13]. Though different types of H5N1 virus clades are circulating in poultry in Bangladesh since 2007, the predominant clade of circulating H5N1 virus was 2.3.2.1 since 2011 [14, 15]. The virus isolates used in these vaccines may not be matched with local influenza A(H5N1) strains perfectly, and that may produce suboptimal protection in commercial chickens. This lower level of immunity may exert evolutionary pressure on the virus, and such newly emerged strains may pose a risk for the emergence of novel strains to the human population [13]. Vaccination has also raised concerns about the possibility of difficult to detect asymptomatic spread of HPAI viruses in chickens with some immunity.
To ensure high levels of herd immunity, 60–80% of H5N1 vaccine coverage is needed in a chicken population [7, 16]. In Bangladesh, there is a need to estimate the vaccination coverage in commercial chicken farms and investigate the impact of the current H5N1 vaccination programs on H5N1 viral shedding to the environment and its possible impacts on the diversity of circulating avian influenza viruses. This study estimated coverage of avian influenza vaccine in poultry farms in Bangladesh and assessed the impact of ongoing H5N1 vaccination on avian influenza virus shedding including H5N1 virus to the environment and examined molecular changes of avian influenza viruses isolated from vaccinated and unvaccinated chickens.
Methods
Study sites
We conducted this study in eight divisions (Dhaka, Chattogram, Rajshahi, Khulna, Barishal, Sylhet, Rangpur, and Mymensingh) of Bangladesh from June 2021 to February 2022. In each division, we identified a sub-district with the largest number of poultry farms were surveyed for baseline data collection (Fig. 1). Samples were collected from selected poultry farms. Poultry farm statistics were collected from the Department of Livestock Services to identify eight sub-districts across eight divisions for the survey.
Fig. 1.
Study sites for baseline data collection of commercial poultry farms
Study design
Initially, we conducted a baseline survey to collect information on H5N1 vaccination coverage of commercial poultry farms. With the local government livestock officials’ help, the study field team visited every commercial poultry farm in the eight selected sub-districts with a large number of commercial poultry farms to collect data. We defined poultry farms as those farms that raise chicken, duck, geese, turkey, pigeon or quail in an intensive farming system (raising a large number of poultry in controlled environments). Commercial chicken farms included commercial layer, commercial broiler, parent stock/breeder, and Sonali chicken. Sonali is the crossbred between Rhode Island Red cocks and Fayoumi hens [17]. Farm size was categorized as scale small (< 1000 poultry), medium (1001–2000 poultry) and large (> 2000 poultry) [17]. The field staff interviewed selected farm owners to collect data using a structured questionnaire. Questionnaire included two sections to collect data on farm demographics and avian influenza vaccination status [including A(H5N1) and A(H9N2)].
Based on the baseline survey data, commercial chicken farms were categorized into two groups; vaccinated and unvaccinated. On the day of the farm visit, those farms that reported vaccinating their current chicken flocks against A(H5N1) or A(H5N2) viruses were categorized as having vaccinated flocks. Commercial chicken farms having only a history of vaccine intake at hatchery or breeder farms against H5N1 or H5N2 was not considered for this study. Chicken flocks with obvious evidence of vaccination history against the H5N1 or H5N2 were prioritized for this study. Considering the above-mentioned criteria, we prepared a final list of vaccinated chicken farms for each of eight sub-districts. Another list was prepared for unvaccinated farms for each sub-district. Those chicken farms that have never administered the vaccines against A(H5N1) or A(H5N2) or A(H9N2) in their current chicken flocks or did not administer vaccine at the hatchery was considered unvaccinated chicken farms.
A total of 400 (200 vaccinated and 200 unvaccinated) farms were identified from eight sub-districts under eight divisions through a two-stage selection process: matching and simple randomization. In each sub-district, total 50 farms (25 vaccinated and 25 unvaccinated) were enrolled for sample and further data collection. In the first step, geographical location (same sub-district) and production systems (layer or broiler) were used to match vaccinated and unvaccinated farms. After farm matching, simple random sampling was used to identify pairs of vaccinated and unvaccinated farm. Every selected farm was visited twice fortnightly for fecal sample collection between December 2021 and February 2022. In each visit, 10 swab sticks were used to collect freshly laid feces from 10 chickens of the selected flock, and the 10 swabs were then combined to prepare a fecal pool sample. A total of 800 fecal pool were collected from 400 selected farms. In vaccinated flock, swabs were taken at least 14 days after vaccination.
Detection and sequencing of avian influenza viruses
All collected samples were tested to detect avian influenza viruses subtypes H5, H7 and H9. The icddr, b (International Centre for Diarrheal Diseases Research, Bangladesh) One health laboratory extracted total RNA from 100 µl of each pool swab specimen from the 800 collected pool samples in VTM, using RNase kit (Qiagen, Cat 74106) according to the manufacturer’s instruction. The extracted RNA was screened for detection of matrix gene of influenza A viruses using the FDA-approved TaqMan qRT-PCR (real-time reverse transcription polymerase chain reaction) assay [18]. Primers and probes specific for matrix (M) gene were included to detect any of the 16 types of avian influenza A viruses. To identify influenza A H5, H7 and H9 subtypes, hemagglutinin (HA) gene specific-primers and probes were used [18]. Full genome sequencing was performed for selected influenza A-positive samples using Illumina MiSeq technology, as previously described [19]. All sequences were uploaded to the Global Initiative on Sharing All Influenza Data.
Phylogenetic analyses
The HA reference nucleotide sequences of H5 and H9 subtypes were obtained from the online repository Global Initiative of Sharing All Influenza Data (GISAID) and Bacterial and Viral Bioinformatics Resource Center (BV-BRC) [20]. Full-length reference sequences of the vaccine strains related to H5 and H9 subtypes of HA genes were included for phylogenic analysis. These sequences were aligned using the ClustalW program within BioEdit software [21]. The alignment was then utilized to construct a phylogenetic tree using the Maximum Likelihood approach using MEGA v.7.0, employing the General Time Reversible (GTR) substitution model with gamma distribution rates [22]. The replicability of the maximum-likelihood tree at each node was assessed by bootstrap analysis of 1000 replications.
Statistical analysis
We performed descriptive analysis to summarize the farm demographics, vaccination status and biosecurity practices of commercial chickens. Flock morbidity and mortality were estimated for vaccinated and unvaccinated farms separately. The proportion of avian influenza viruses (AIVs) and AIV (H5) for vaccinated and unvaccinated flocks were estimated separately. Chi-square test was performed to estimate the significance difference of different avian influenza viruses subtypes occurrence between vaccinated and unvaccinated flock. We estimated odds ratios (OR) and 95% confidence intervals (CI) to identify associations between vaccination status and AIV (H5) detection. The variables with a p-value of < 0.05 in univariate analysis were selected for multivariate analysis. Backward stepwise selection of variables with a significance level of 0.05 was used to construct final models. Variables were retained or removed from the model after considering the p-value of < 0.05. We estimated the adjusted odds ratios (aOR) using multivariate logistic regression, combining all significant variables.
Results
Demographic characteristics of surveyed poultry farms
A total of 5092 farm owners were interviewed from eight sub-districts under eight divisions to collect baseline information. Majority of the farms were enrolled from Sreepur (18%, n = 898). Majority of the enrolled farms reared layer chicken (50%, n = 2565), followed by broiler chicken (35%, n = 1802) and Sonali chicken (10%, 486). Most of the farms (52%, n = 2664) were small in size having less than 1000 poultry. According to the farmers self-report, more than 75% (n = 3876) of the farms were not registered by Government and 46% of the farmers had experience for chicken farming from 1 to 5 years. The average chicken population per farm was comparatively higher in layer farms (n = 1862) than broiler (1099) and Sonali (1248) farms (Table 1).
Table 1.
Demographic characteristics of poultry farms, Bangladesh, June 2021 to February 2022 (N = 5092)
| Characteristics | n (%) | 95% CI |
|---|---|---|
| Sub-district wise poultry farms enrollment | ||
| Sreepur | 898 (18) | 17–19 |
| Fulbaria | 894 (18) | 17–19 |
| Fatikchhari | 647 (13) | 12–14 |
| Sundarganj | 616 (12) | 11–13 |
| Paba | 576 (11) | 10–12 |
| Kaliganj | 549 (11) | 10–12 |
| Nesarabad | 521 (10) | 9–11 |
| Golapganj | 391 (8) | 7–8 |
| Types of poultry farms | ||
| Broiler chicken | 1802 (35) | 34–37 |
| Layer chicken | 2565 (50) | 49–52 |
| Sonali chicken | 486 (10) | 9–10 |
| Duck and Geese | 189 (4) | 3–4 |
| Native chicken | 22 (1) | 0–1 |
| Others | 28 (1) | 1–2 |
| Farm size | ||
| Small (≤ 1000 poultry) | 2664 (52) | 51–54 |
| Medium (1001–2000 poultry) | 1633 (32) | 31–33 |
| Large (> 2000 poultry) | 795 (16) | 15–17 |
| Number of registered farms by Government | ||
| Yes | 1216 (24) | 23–25 |
| No | 3876 (76) | 75–77 |
| Farm longevity | ||
| ≤ 3 years | 1381 (27) | 26–28 |
| 4–6 years | 1386 (27) | 26–28 |
| 7–9 years | 756 (15) | 14–16 |
| 10–12 years | 844 (17) | 16–18 |
| ≥ 13 years | 725 (14) | 13–15 |
| Farming experience by farmers | ||
| 1–5 years | 2352 (46) | 45–48 |
| 6–10 years | 1616 (32) | 30–33 |
| 11–20 years | 1026 (20) | 19–21 |
| > 20 years | 98 (2) | 1–2 |
| Average number broiler chicken per farms, mean, (SD, range) | 1099 (± 1587, 100-50000) | |
| Average number layer chicken per farms, mean, (SD, range) | 1862 (± 2456, 79-60000) | |
| Average number Sonali chicken per farms, mean, (SD, range) | 1248 (± 1215, 100-10000) | |
| Average number duck per farms, mean, (SD, range) | 251 (± 257, 24-2600) | |
| Average numbers of farm workers per farm, mean, (SD, range) | 2.3 (± 1.02, 1–24) | |
Vaccination coverage against H5N1 virus
Overall, more than 95% of the poultry farms initially surveyed reported using some type of vaccines. Vaccine coverage varied by vaccine type (e.g. avian influenza, Newcastle disease, fowl pox and Marek’s) and production type (e.g. broiler, layer and Sonali). Overall, 1284 (25%) farms administered vaccine against AIV/H5 virus in their current chicken flocks. Among the vaccinated farms, 1276 (99%) were layer chicken farms. Of the 2565 surveyed layer chicken farms, 1276 (50%) farms used vaccine against H5N1 virus in their current batch. The vaccination against H5N1 virus in broiler and Sonali chicken farms was extremely low (n = 8). A substantial number of layer chicken farms (24%, n = 607) reported of using vaccine against H9N2 virus (Table 2).
Table 2.
History vaccination in poultry farms, Bangladesh, June 2021 to February 2022 (N = 5092)
| Vaccination characteristics | Number of farms performed vaccination | p value | |
|---|---|---|---|
| Yes n(%) |
No n(%) |
||
| Previously used vaccines to poultry | |||
| Administered any vaccine | 4892 (96) | 200 (4) | < 0.001 |
| Administered vaccine against avian influenza | 1311 (26) | 3781 (74) | |
| Administered vaccine against Newcastle disease | 4770 (94) | 322 (6) | |
| Administered vaccine against infectious bursal disease | 4501 (88) | 591 (12) | |
| Administered vaccine against fowl pox | 2310 (45) | 2782 (55) | |
| Administered vaccine against fowl cholera | 2310 (45) | 2782 (55) | |
| Administered vaccine against Marek’s | 2243 (44) | 2849 (56) | |
| Administered vaccine against duck plague | 154 (3) | 4938 (97) | |
| Administered vaccine against H5N1 virus to their current commercial chicken flock | |||
| Broiler | 2 (1) | 1802 (100) | < 0.001 |
| Layer | 1276 (50) | 1289 (50) | |
| Sonali | 6 (1) | 480 (99) | |
| Administered vaccine against H9N2 virus to their current commercial chicken flock | |||
| Broiler | - | 1802 (100) | < 0.001 |
| Layer | 607 (24) | 1958 (76) | |
| Sonali | 2 (1) | 484 (100) | |
Characteristics of sampled chicken farms
A total of 400 chicken farms (200 vaccinated and 200 unvaccinated) were selected from eight divisions. Most of vaccinated (49%, n = 197) and unvaccinated (49%, n = 195) farms were layer chicken farms, and the remainder were Sonali farms. Three different categories of farms such as small (n = 151), medium (n = 136) and large (n = 113) scale were included in vaccinated and unvaccinated arms. Most of the chickens in both vaccinated (41%, n = 164) and unvaccinated (37%, n = 149) arms were aged less than 12 months. Average number of layer chickens per farm in vaccinated and unvaccinated arms was 2454 and 1550, respectively. The average number of Sonali chicken per farm in vaccinated and unvaccinated arms was 1683 and 1316, respectively (Table 3).
Table 3.
Demographic characteristics of sampled poultry farms, Bangladesh, June 2021 to February 2022 (N = 400)
| Characteristics | Vaccinated farms n (%) |
Unvaccinated farms n (%) |
|---|---|---|
| Division wise poultry farms enrollment | ||
| Dhaka | 25 (13) | 25 (13) |
| Chattogram | 25 (13) | 25 (13) |
| Sylhet | 25 (13) | 25 (13) |
| Rajshahi | 25 (13) | 25 (13) |
| Khulna | 25 (13) | 25 (13) |
| Barishal | 25 (13) | 25 (13) |
| Rangpur | 25 (13) | 25 (13) |
| Mymensingh | 25 (13) | 25 (13) |
| Types of poultry farms | ||
| Layer chicken | 197 (49) | 195 (49) |
| Sonali chicken | 3 (1) | 5 (1) |
| Farm size | ||
| Small (≤ 1000 poultry) | 64 (16) | 87 (22) |
| Medium (1001–2000 poultry) | 64 (16) | 72 (18) |
| Large (> 2000 poultry) | 72 (18) | 41 (10) |
| Farm longevity | ||
| ≤ 12 months | 164 (41) | 149 (37) |
| 13–24 months | 35 (9) | 47 (12) |
| ≥ 25 months | 1 (1) | 4 (1) |
| Farming experience by farmers | ||
| 1–5 years | 64 (16) | 70 (18) |
| 6–10 years | 66 (17) | 73 (18) |
| 11–20 years | 60 (15) | 54 (14) |
| > 20 years | 10 (3) | 3 (1) |
| Average number layer chicken per farms, mean, (SD, range) | 2454 (± 3983, 200-50000) | 1550 (± 1412, 71-10000) |
| Average number Sonali chicken per farms, mean, (SD, range) | 1683 (± 1013, 180–3000) | 1316 (± 1176, 550–2500) |
Detection, identification and sequencing of AIVs in vaccinated and unvaccinated farms
Of the 800 fecal pools, avian influenza A viral RNA was detected in 24 (3%) pooled samples. Overall, 21 different farms (5%) had at least one fecal pool sample test positive for avian influenza. Samples from three farms were confirmed for H5 subtype specific viral RNA, 17 farms were confirmed for H9 subtype specific viral RNA and sample from one farm remained unsubtypable (not positive for H5, H7 or H9). Of the three H5 positive farms, one had history of vaccination against H5N1 virus. Among the 17 H9 positive farms, seven farms had history of vaccination against H5N1 virus (Table 4). A total of 28 avian influenza positive samples were sequenced; two of them were identified as H5N1 subtype and 26 samples were H9N2 subtype (Fig. 2, Supp. Figures 1 and 2).
Table 4.
Farm level avian influenza virus detection in chickens from vaccinated and unvaccinated farms, Bangladesh, January 2022 to February 2022 (N = 400)
| Divisions | H5N1 Vaccination status | Number of farms tested | No. (%) of farms positive for influenza A | No. (%) of farm positive for influenza A/H5 | No. (%) of farms positive for influenza A/H9 |
|---|---|---|---|---|---|
| Dhaka | Vaccinated | 25 | 2 | - | 2 |
| Unvaccinated | 25 | 1 | - | 1 | |
| Chattogram | Vaccinated | 25 | - | - | - |
| Unvaccinated | 25 | - | - | - | |
| Sylhet | Vaccinated | 25 | - | - | - |
| Unvaccinated | 25 | 2 | - | 2 | |
| Rajshahi | Vaccinated | 25 | 3 | 1 | 3 |
| Unvaccinated | 25 | 6 | 2 | 5 | |
| Khulna | Vaccinated | 25 | 1 | - | 1 |
| Unvaccinated | 25 | - | - | - | |
| Barishal | Vaccinated | 25 | - | - | - |
| Unvaccinated | 25 | 1 | - | 1 | |
| Rangpur | Vaccinated | 25 | 2 | - | 1 |
| Unvaccinated | 25 | 1 | - | 1 | |
| Mymensingh | Vaccinated | 25 | 1 | - | 1 |
| Unvaccinated | 25 | 1 | - | 1 | |
| Total | 400 | 21 (5%) | 3 (1%) | 19 (5%) |
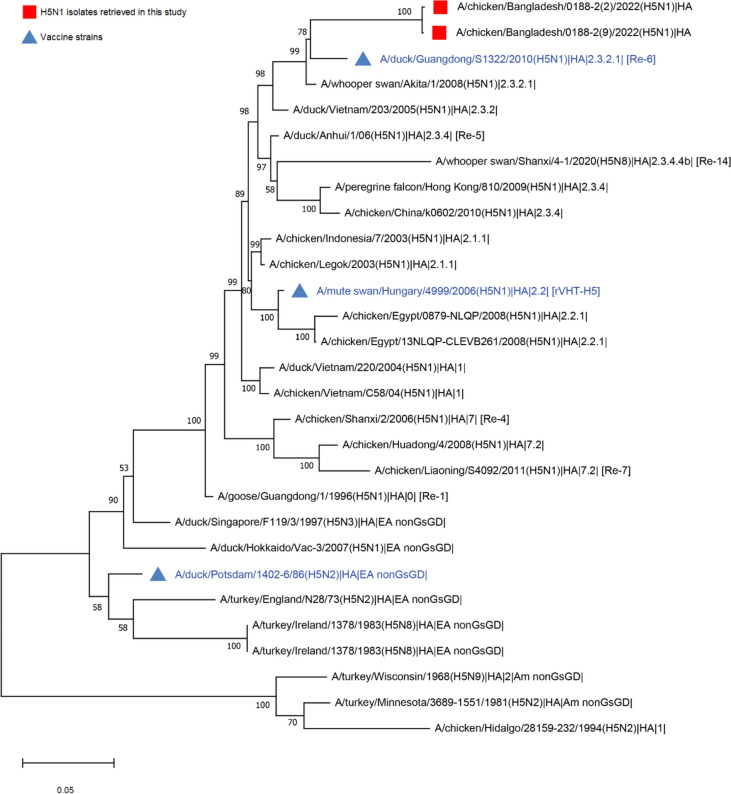
Fig. 2.
The phylogenetic tree based on the maximum likelihood approach depicts the relationship of H5N1 isolates sequenced in this study with reference sequences of vaccine strains representing different clades. The analysis involved 29 sequences, with a final dataset comprising a total of 1672 aligned positions. Red squares represent the isolates retrieved in this study, whereas blue triangles indicate the vaccine strains used or licensed for use in Bangladesh. The tree is drawn to scale, indicating a substitution rate of 0.050 per nucleotide position
Phylogenetic analysis
Phylogenetic analysis of the H5 sequences indicated that the viruses belonged to clade 2.3.2.1 and formed a close cluster together with the vaccine strain A/duck/Guangdong/S1322/2010 (H5N1) [Re-6] but not closely related with other vaccine strains (Fig. 1). Phylogenetic analysis of the H9N2 strains identified in this study, when compared with the vaccine strain (Ceva, a G1-like Middle East H9N2 isolate), suggests that the isolates formed distinct clusters, indicating their genetic diversity (Supp. Figure 2).
Association between avian influenza virus detection and vaccination status
In univariate analyses, chicken farms that had a history of vaccination against H5N1 virus (OR 0.49, 95% CI:0.44–0.55) were less likely to be positive for avian influenza A/H5 viral RNA compared with farms that did not have these characteristics. In multivariate regression analysis, chicken farms that administered vaccine against H5N1 to chicken (aOR 0.39, 95% CI: 0.32–0.48) was found protective for the detection of H5N1 viral RNA. In the multivariate regression model, clustering due to geographical locations of farms was adjusted along with other demographics and biosecurity variables (Table 5).
Table 5.
Farm level avian influenza subtype H5 detection, H5N1/H9N2 vaccination status and other biosecurity measures January 2022 to February 2022 (N = 400)
| Variables | No. (%) farms | No. (%) farms positive for avian influenza A/H5 | OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| H5N1 Vaccination status | ||||||
| No | 200 | 2 | Ref. | |||
| Yes | 200 | 1 | 0.49 (0.45–0.55) | < 0.001 | 0.39 (0.32–0.48) | < 0.001 |
| H9N2 Vaccination status | ||||||
| No | 349 | 3 | Ref. | |||
| Yes | 51 | 0 | Undefined | |||
| Types of chicken | ||||||
| Exotic layer chicken | 393 | 3 | Ref. | |||
| Sonali | 7 | - | Undefined | - | ||
| Farm size | ||||||
| Small (≤ 1000 chicken) | 151 | - | Ref. | |||
| Medium (1001–2000 chicken) | 136 | 3 | Undefined | |||
| Large (> 2000 chicken) | 113 | - | Undefined | |||
| Presence of fence around farm | ||||||
| No | 241 | 3 | Ref. | |||
| Yes | 159 | 0 | Undefined | |||
| Cleaning farm premises | ||||||
| Daily | 72 | Ref. | ||||
| Every alternative day | 193 | 2 | Undefined | |||
| Weekly | 49 | Undefined | ||||
| Fortnightly | 17 | 1 | Undefined | |||
| Monthly | 69 | Undefined | ||||
| Disinfection inside farm premises | ||||||
| Daily | 53 | Ref. | ||||
| Every alternative day | 120 | Undefined | ||||
| Weekly | 187 | 2 | Undefined | |||
| Fortnightly | 11 | Undefined | ||||
| Monthly | 22 | 1 | Undefined | |||
| No disinfection | 7 | Undefined | ||||
| Drainage system | ||||||
| Nonfunctional or absent | 246 | 3 | Ref. | |||
| Functional | 154 | 0 | Undefined | |||
| Slaughtering chicken within farm yard | ||||||
| No | 249 | 1 | Ref. | |||
| Yes | 151 | 2 | 3.33 (2.42–4.56) | < 0.001 | 3.36 (2.36–4.77) | < 0.001 |
| Using separate shoes during entering inside farm | ||||||
| No | 56 | 1 | Ref. | |||
| Yes | 344 | 2 | 0.32 (0.15–0.67) | 0.003 | 0.34 (0.14–0.85) | < 0.022 |
| Raising multiple poultry species adjacent to the farm | ||||||
| No | 396 | 3 | Ref. | |||
| Yes | 4 | 0 | Undefined | |||
| Access within farm yard by outside transports | ||||||
| No | 89 | 0 | Ref. | |||
| Yes | 311 | 3 | Undefined | |||
Discussion
This study explored the H5N1 vaccination coverage in eight sub-districts under eight divisions in Bangladesh. The vaccination coverage against H5N1 virus in their current chicken flock was below the optimal standard (60–80% vaccination coverage). The low level of vaccination coverage reported by this study raised a concern about the effectiveness of ongoing vaccination programme for significant reduction of H5N1 virus transmission in poultry population, as previous studies indicate that achieving 60–80% vaccination coverage is necessary to maintain high levels of flock immunity [7, 16, 23]. Among H5N1 endemic countries, Hong Kong reported the highest vaccination coverage at 86%, followed by Egypt at 70%, Vietnam at 52%, Mongolia at 51%, and China at 47% between 2002 and 2010 [24]. To our knowledge, this is the first study to assess the H5N1vaccination coverage. Stakeholders in commercial chicken production should focus on enhancing H5N1 vaccination coverage. Additionally, it is crucial for policymakers and health officials in both human and animal health to ensure that H5N1 vaccination does not introduce any further risks to human health.
The effectiveness of vaccines against avian influenza strains relies on strong cross-reactivity with the cocirculating avian influenza virus strains [25]. Interestingly, all H5 vaccines used in Bangladesh were imported by local pharmaceutical companies and none of these vaccines contained viruses isolated locally [12, 13]. The mismatch between field viruses and vaccine strains may produce suboptimal level of immunity that trigger genetic changes and the evolution of new avian influenza viruses that may pose a risk for the emergence of novel strains to the human population. Phylogenetic analysis of the H5 sequences revealed that the viruses belonged to clade 2.3.2.1 and formed a close cluster together with the vaccine strain A/duck/Guangdong/S1322/2010 (H5N1) [Re-6]. The decline of clade 2.2 and EA-nonGsGD after administration of rHVT-H5 vectored vaccine and Potsdam/1986 H5N2 inactivated vaccine, respectively indicates a significant reduction in H5N1 lineage diversity in vaccinated poultry [26]. However, two H5N1 isolates of clade 2.3.2.1 in this study survived post-vaccination of RE-6 inactivated vaccine containing clade 2.3.2.1 suggesting the possibility of selection and persistence of the viruses of this clade. Evolutionary changes in A(H5N1) viruses were also observed in countries using vaccines against A(H5N1) when compared to countries that never used A(H5N1) vaccine in poultry [8]. In Vietnam, low protection levels were detected in ducks and high viral shedding occurred more than 7 days after an A(H5N1) challenge with mortality [9]. In Egypt, surveillance detected continuous circulation of avian influenza viruses including the A(H5N1) viruses in vaccinated commercial and backyard poultry [10]. Another study in Egypt revealed imported vaccine-mediated antigenic drift evolution of influenza A (H5N1) viruses in vaccinated commercial chickens [11].
Over the study period, we detected only three farms tested positive for HPAI virus (H5N1). This result indicates that farm level H5N1 circulation was very low in both vaccinated and unvaccinated farms during the sampling period. The ongoing live bird market based avian influenza surveillance detected comparatively higher number of poultry (3%) positive for H5 subtype during 2021–2022 (personal communication). This difference suggests that live bird markets may be more likely to sustain circulation of AIVs than farms. The low detection of H5N1 virus found in this study limit our efforts to evaluate the effectiveness of avian influenza vaccine.
Our research results suggested that layer chicken farmers demonstrated a greater inclination to administer the H5N1 vaccine in comparison to those who raise broilers and Sonali chickens. Like our study findings, difference in vaccination coverage was reported in three different countries during 2004 to 2009. In Egypt, broiler chicken (78%) was mostly vaccinated than layer chicken (32%) [24]. In Pakistan, the highest H5 vaccination coverage was recorded in breeder chicken (26%) compared to layer chicken (3%) and no vaccination to broiler chicken [24]. On the contrary, the highest vaccination coverage was reported by Vietnam in meat duck (90%) than broiler chicken (52%) [24]. The high vaccination coverage was observed in layer chicken farms in this study could be due to the significant investment in layer chicken farms, longer lifespans of layer chicken compared to broilers, and heightened awareness among layer farmers compared to those raising broiler and Sonali chickens.
Bangladesh has reported only a few H5N1 outbreaks in poultry since 2013, compared to the period between 2007 and 2012 [12, 27]. This low number of H5N1 outbreaks could be due the implementation of countrywide vaccination in commercial chicken farms. In contrast, the ongoing live bird market based avian influenza surveillance has consistently detected AIVs in poultry from 2007 to the present day. These inconsistent outcomes have raised questions about the efficacy of existing H5N1 vaccination efforts, the reliability of outbreak reporting systems, and the role of live bird markets in sustaining the transmission of avian influenza viruses.
This study had several limitations. Only commercial layer and Sonali chicken farms were sampled to detect viral shedding. We did not find any broiler farms that had history of H5N1 vaccination during sampling. Therefore, this study findings only represent the effectiveness of H5N1 vaccination in layer and Sonali chicken. Detection of very low number of farms for H5N1 virus limit our efforts to evaluate the effectiveness of avian influenza vaccine.
Conclusions
The H5N1 vaccination coverage was below standard and limited to only layer chickens. The detection of the H5N1 virus was lower in both vaccinated and unvaccinated chicken flocks, which constrained the study’s ability to evaluate the vaccine’s effectiveness in reducing HPAI H5N1 viral shedding. Phylogenetic analysis suggests Re-6 vaccine can protect current circulatory H5N1 virus better than other available vaccines. Regular monitoring is needed to assess the vaccination coverage, impact of ongoing avian influenza vaccination on viral shedding, genetic reassortment and emergence of new influenza strains of public health importance.
Supplementary Information
Supplementary Material 1: Supplementary Fig. 1. The phylogenetic tree based on the maximum likelihood approach shows the relationship of H9N2 isolates sequenced in this study, as indicated by red squares. The sequences of previously reported H9N2 viruses from Bangladesh are indicated by blue squares. The analysis involved a total of 64 sequences, with a final dataset comprising 1682 aligned positions. The tree is drawn to scale, indicating a substitution rate of 0.050 per nucleotide position. Supplementary Fig. 2. The phylogenetic tree based on the maximum likelihood approach illustrates the relationship between the H9N2 isolates of this study and the commercially available H9N2 vaccines, which are developed from specified vaccine strains. The H9N2 vaccines currently marketed in Bangladesh are denoted by blue triangles. This analysis incorporated 41 sequences in total, resulting in a final dataset of 1469 aligned positions. The tree is drawn to scale, representing a substitution rate of 2.00 per nucleotide position.
Acknowledgements
We express our heartfelt thanks to Dr. William Davis, Dr. Todd Davis and Muhammad Manwar Morshed Hemel for their valuable inputs during protocol development, implementation of this study and writing manuscript. We gratefully acknowledge icddr, b core donors (Government of Bangladesh and Canada) for their unrestricted support.
Authors’ contributions
S.C., M.E.H., S.K.B., S.G., M.R., F.C and M.Z.R. participated in conceptualization, methodology, laboratory work, and preparation of the manuscript. S.C., M.E.H., S.K.B., S.G., P.K.G., M.R., F.C and M.Z.R. participated in methodology, supervision and manuscript editions. S.C., M.E.H, R.H., M.M., M.M.H., J.Q.A., A.S and M.Z.R. contributed to the supervision of laboratory work, data analysis and manuscript editions. All authors have read and approved the final manuscript for publication.
Funding
This research study was funded by the Centers for Disease Control and Prevention, USA (cooperative agreement no. 1U01GH001207-01).
Data availability
Data are available and shared from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This protocol was approved by the Institutional Review Board of icddr, b (PR-20140). All the respondents above 18 years of age were asked to provide informed, written consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. 2018.http://www.who.int/influenza/human_animal_interface/2018_03_02_tableH5N1.pdf?ua=1.
- 2.WHO. Analysis of recent scientific information on avian influenza A(H7N9) virus. 2017. https://www.who.int/influenza/human_animal_interface/avian_influenza/riskassessment_AH7N9_201702/en/.
- 3.OIE. Update on avian influenza in animals (types H5 and H7). 2018. http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2018/.
- 4.Yee KS, Carpenter TE, Cardona CJ. Epidemiology of H5N1 avian influenza. Comp Immunol Microbiol Infect Dis. 2009;32(4):325–40. [DOI] [PubMed] [Google Scholar]
- 5.Spackman E, Swayne DE. Vaccination of gallinaceous poultry for H5N1 highly pathogenic avian influenza: current questions and new technology. Virus Res. 2013;178(1):121–32. [DOI] [PubMed] [Google Scholar]
- 6.Swayne DE. Impact of vaccines and vaccination on global control of avian influenza. Avian Dis. 2012;56(4s1):818–28. [DOI] [PubMed] [Google Scholar]
- 7.Savill NJ, et al. Silent spread of H5N1 in vaccinated poultry. Nature. 2006;442(7104):757. [DOI] [PubMed] [Google Scholar]
- 8.Cattoli G, et al. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine. 2011;29(50):9368–75. [DOI] [PubMed] [Google Scholar]
- 9.Cha RM, et al. Suboptimal protection against H5N1 highly pathogenic avian influenza viruses from Vietnam in ducks vaccinated with commercial poultry vaccines. Vaccine. 2013;31(43):4953–60. [DOI] [PubMed] [Google Scholar]
- 10.Hafez M, et al. Avian influenza H5N1 virus infections in vaccinated commercial and backyard poultry in Egypt. Poult Sci. 2010;89(8):1609–13. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Moneim AS, Afifi MA, El-Kady MF. Genetic drift evolution under vaccination pressure among H5N1 Egyptian isolates. Virol J. 2011;8(1): 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rimi NA, et al. A decade of avian influenza in Bangladesh: where are we now? Trop Med Infect Dis. 2019;4(3):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon J-H, et al. Genetic evolution and Transmission Dynamics of Clade 2.3. 2.1 a highly pathogenic avian influenza A/H5N1 viruses in Bangladesh. Virus Evolution; 2020. [DOI] [PMC free article] [PubMed]
- 14.Gerloff NA, et al. Multiple reassortment events among highly pathogenic avian influenza A (H5N1) viruses detected in Bangladesh. Virology. 2014;450:297–307. [DOI] [PubMed] [Google Scholar]
- 15.Barman S, et al. Continuing evolution of highly pathogenic H5N1 viruses in Bangladeshi live poultry markets. Emerg Microbes Infections. 2019;8(1):650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouma A, et al. Estimation of transmission parameters of H5N1 avian influenza virus in chickens. PLoS Pathog. 2009;5(1):e1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury S, et al. Antibiotic usage practices and its drivers in commercial chicken production in Bangladesh. PLoS ONE. 2022;17(10): e0276158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC, Centers for Disease Control and Prevention Laboratory Support for Influenza Surveillance (CLSIS). CDC, Atlanta. 2013.
- 19.Shepard SS, et al. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genomics. 2016;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson RD, et al. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023;51(D1):D678-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. InNucleic acids symposium series. 1999;41(41):95–98.
- 22.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiensin T, et al. Transmission of the highly pathogenic avian influenza virus H5N1 within flocks during the 2004 epidemic in Thailand. J Infect Dis. 2007;196(11):1679–84. [DOI] [PubMed] [Google Scholar]
- 24.Swayne D, et al. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev Sci Tech-OIE. 2011;30:839. 3. [DOI] [PubMed] [Google Scholar]
- 25.Kim SM, et al. Vaccine efficacy of inactivated, chimeric hemagglutinin H9/H5N2 avian influenza virus and its suitability for the marker vaccine strategy. J Virol. 2017;91(6):01693–01616. 10.1128/jvi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon J-H, et al. Genetic evolution and transmission dynamics of clade 2.3. 2.1 a highly pathogenic avian influenza A/H5N1 viruses in Bangladesh. Virus Evol. 2020;6(2):veaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury S, et al. The pattern of highly pathogenic avian influenza H5N1 outbreaks in South Asia. Trop Med Infect Dis. 2019;4(4):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Fig. 1. The phylogenetic tree based on the maximum likelihood approach shows the relationship of H9N2 isolates sequenced in this study, as indicated by red squares. The sequences of previously reported H9N2 viruses from Bangladesh are indicated by blue squares. The analysis involved a total of 64 sequences, with a final dataset comprising 1682 aligned positions. The tree is drawn to scale, indicating a substitution rate of 0.050 per nucleotide position. Supplementary Fig. 2. The phylogenetic tree based on the maximum likelihood approach illustrates the relationship between the H9N2 isolates of this study and the commercially available H9N2 vaccines, which are developed from specified vaccine strains. The H9N2 vaccines currently marketed in Bangladesh are denoted by blue triangles. This analysis incorporated 41 sequences in total, resulting in a final dataset of 1469 aligned positions. The tree is drawn to scale, representing a substitution rate of 2.00 per nucleotide position.
Data Availability Statement
Data are available and shared from the corresponding author upon reasonable request.