Abstract
Background
Chronic non-cancer pain (CNCP) is a common condition worldwide. The disease burden is influenced not only by pain itself, but also by psychiatric co-morbidities, which aggravate symptoms, generally negatively influence therapies, and may thereby lead to frustration, resignation, or withdrawal. A growing body of evidence suggests that sex and gender aspects influence CNCP management as the experience of pain, the emotions associated with it, and the expression of pain may differ between women and men. In addition, doctor-patient communication is known to be influenced by gender stereotypes. Despite there being evidence on such differences, current guidelines do not consider sex- and gender-sensitive approaches. In order to examine how to adequately address the diversity of the experience and processing of pain in patients of differing sex and gender, the GESCO study aims at developing and pilot testing a sex- and gender-sensitive intervention for patients with CNCP receiving long-term opioid therapy (LTOT) in primary care.
Methods
The development process is designed in accordance with the first two phases of the UK Medical Research Council. Phase I will iteratively explore, develop, and pilot the intervention’s modules using literature searches, interviews, and workshops involving stakeholders and experts. Phase II will pilot-test the novel intervention in a sample of 40 patients with CNCP under LTOT from ten general practices using an effectiveness-implementation hybrid design including a mixed-methods process evaluation focusing on implementation strategy criteria and a single-arm, pre-post comparison to determine preliminary effects in preparation for a larger effectiveness trial. The intervention will combine in-person educational sessions for general practitioners and tools to be used in patient care.
Discussion
The intervention aims to improve CNCP management in primary care by empowering practitioners to reflect on their attitudes towards pain and stereotypes. Besides sex and gender aspects, awareness of other factors that might affect the care process, such as age, social conditions, or culture, is also promoted. The intention is to develop a comprehensive care concept for CNCP that considers aspects relevant for sex- and gender-sensitive care which are transferrable to other health care fields as well.
Trial registration
German Clinical Trial Register DRKS00029980.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40814-024-01564-7.
Keywords: Chronic pain, Opioids, Gender role, Sex, Primary care
Background
Chronic non-cancer pain (CNCP) is a common condition worldwide, negatively affecting individuals, families, and communities as well as national economies [1–4]. Opioid therapy can significantly improve CNCP symptoms but may also pose a problem in itself [5]. Germany is among the countries with the highest per capita consumption of opioids for CNCP worldwide [6]: in 2012, 1.3% of insured persons received long-term opioid therapy (LTOT). The disease burden in patients with CNCP is influenced not only by pain itself, but also by psychiatric co-morbidities, which commonly aggravate symptoms and are known to hamper therapy [7, 8]. In everyday care, insufficient success of CNCP treatment often results in frustration for both the patient and the therapist and may lead to resignation and withdrawal on one or both sides [9, 10], frequently associated with opioid misuse [9, 11, 12].
A growing body of evidence suggests that CNCP management and co-morbidities are subject to influences associated with sex and gender aspects [13–15]. Gender is a concept considering social, environmental and situational connotations, identity and the role of a person whereas sex refers to the biological function. However, sex and gender do influence each other, which frequently impairs a differentiation between sex- or gender-specific aspects [16]. In medicine, sex and gender differences are well described for many conditions where gender differences in pain perception and coping with pain have been described in animals and humans [17–19]. In pharmacology, sex differences have been described for the pharmacokinetics as well as the pharmacodynamics of many frequently used drugs [20, 21]. Among them are opioids, where a body of evidence supports the hypothesis that gonadal hormones influence and determine sex-specific differences in pain and opioid-associated effects [22, 23].
In the context of chronic pain, sex and gender aspects are relevant not only regarding different prevalences of co-morbidities, but also in terms of experiencing and processing chronic pain itself. Among others, studies report differences regarding pain-related negative emotions such as anxiety and frustration [24], more detailed reporting of negative experiences with their physicians among women [25], and a higher prevalence of childhood trauma and family conflict in women [15]. It is also known that male and female patients with CNCP differ in how they verbally and non-verbally express pain [26] and that health care professionals’ communication is influenced by gender stereotypes [10, 27, 28].
Even though there is evidence on differences between men and women, current clinical guidelines focus on critically reviewing the therapy of patients with CNCP, examining whether opioid prescriptions are adequate and which alternatives might be used instead, but they do not consider any gender-sensitive approaches [29–32].
Despite the recognized importance of gender in doctor-patient communication, most communication skills assessment instruments in medical education neglect this factor, with only a minority incorporating gender-related content. To improve communication training for medical professionals, clearer criteria and purposes for integrating gender considerations into assessment practices are needed [33]. Doing this, it has to be taken into account that beyond the existing research on gender topics, there are still gaps in knowledge regarding the actual magnitude of gender disparities in pain care as well as on which specific interventional strategies might help to adequately address these disparities. Thus, there is a need to participatory develop more targeted group-specific care concepts for patients with CNCP on LTOT that consider a variety of sex- and gender-sensitive approaches. Such approaches should not include stereotypical standard interventions for women and men, respectively, but should increase awareness for stereotypical differences and also enable physicians to address patients in light of other individual factors such as biological, cultural or psychosocial background, which are known to be relevant for CNCP management as well [34]. As the results of dyad research suggest [35], this requires an intervention that includes elements of reflection on the physicians’ subjective attitudes towards pain and gender and their role as practitioners, in addition to improving their (sex- and gender-sensitive) communicative competencies [36]. This approach has the potential to benefit patients by fostering greater empathy and understanding in doctor-patient interactions, ultimately leading to more tailored and appropriate care.
Objectives
The aims of the study are twofold: The first is to develop a novel gender-sensitive care for chronic non-cancer pain patients receiving long-term opioid therapy (GESCO) intervention to support individuals with CNCP receiving LTOT in primary care. In this step, the elements of the GESCO intervention including implementation strategies required to apply the intervention in the care of patients with CNCP will be developed. The second is to determine the feasibility of this intervention from the perspective of patients with CNCP and their general practitioners (GPs). This feasibility study aims to identify potential refinements to the intervention’s contents and design prior to a larger effectiveness trial, and to determine preliminary effects to estimate effect sizes for a larger trial [37, 38].
Methods/design
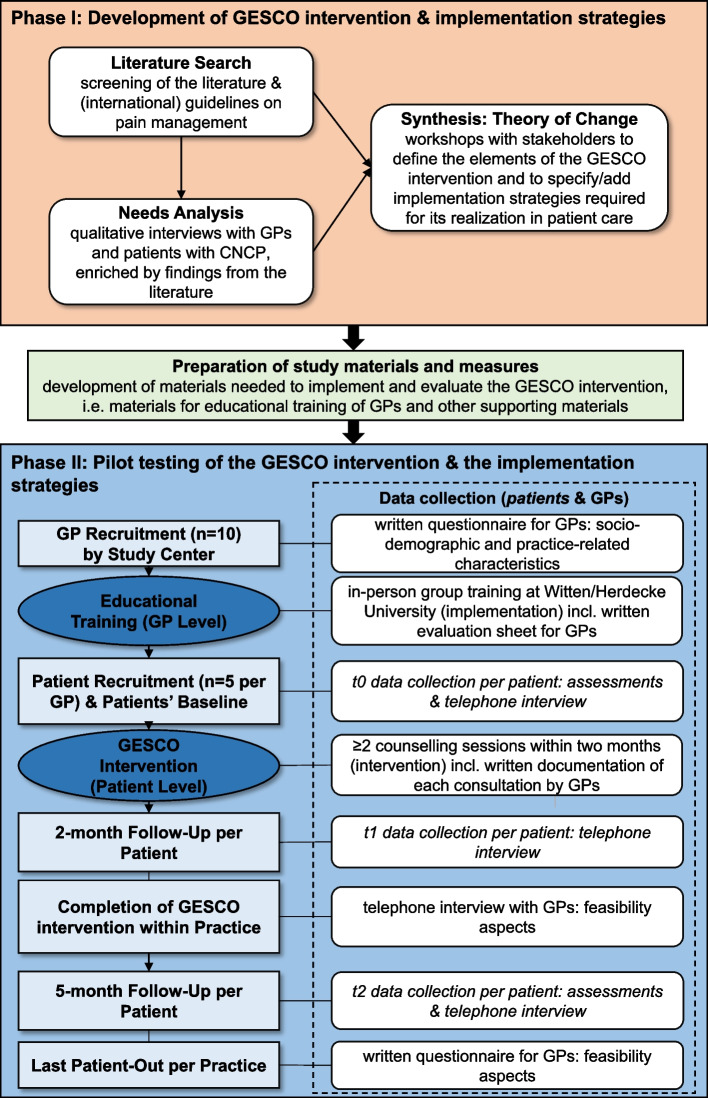
The GESCO intervention will be developed and pilot tested in terms of feasibility criteria in accordance with the two first phases of the UK Medical Research Council (MRC) Framework for developing complex interventions [39, 40]: First, a development and modeling process will be applied in order to iteratively explore, develop, and evaluate the contents and single modules of the sex- and gender-sensitive GESCO intervention by involving stakeholders and experts (phase I). Second, an exploratory mixed-methods study in a clinical sample of 40 patients with CNCP receiving LTOT, who get managed by ten primary care practices that will be educated previously, will be conducted in order to pilot-test the intervention and determine whether the intervention is feasible for a future effectiveness study (phase II) [38, 41]. The study conduct is visualized in Fig. 1.
Fig. 1.
Study flow of the GESCO study
Reporting of the study refers to the SPIRIT checklist [42], but was adapted for reporting the protocol of a feasibility study considering the CONSORT statement for pilot and feasibility trials [41, 43].
Phase I: development of the GESCO intervention and implementation strategies
Study design
Starting with a theoretical phase, the existing literature will be screened for systematic reviews and original studies focusing on sex- and gender-specifics in CNCP related to pharmacological and non-pharmacological treatment, co-morbidities, patients’ needs, and physician–patient communication, as well as social dimensions. Additionally, national and international guideline recommendations for CNCP treatment will be screened to explore whether they provide any sex- and/or gender-specific recommendations. Based on the findings identified in the literature, patients with CNCP and GPs will be interviewed to assess their needs in the light of the current knowledge.
As a starting point for intervention design, the results of the literature review and stakeholder needs assessments (interviews with GPs and patients with CNCP) will be synthesized into a theory of change [44, 45]: During a participatory workshop with patients, physicians and experts (such as psychologists or sociologists), a long-term goal for the intervention will be defined. Based on the long-term goal, short and intermediate intervention outcomes, activities and inputs will be collected. In a second consecutive workshop the results from the first workshop will be specified—including strategies regarding implementation and sustainment of the intervention. Based on the theory of change, intervention materials and documents will be developed and pre-tested in general practices. The evaluation design will also follow the theory of change.
Study setting and eligibility criteria
In order to consider GPs’ and patients’ needs alike, both GPs managing patients with CNCP and patients with CNCP themselves aged ≥ 18 years who have received opioid therapy for at least 3 months will be involved in the development of the intervention. As interviews and workshops will be conducted in German, all participants have to possess sufficient German language skills.
Sample size and recruitment
A minimum of six GPs and six patients with CNCP will be recruited for the interviews. The interviews will be carried out following the concept of information power until we have sufficient information power for the analysis of our research questions as well as for the generated quality of the dialog [46].
GPs will personally be invited to participate in an interview on their needs to adequately manage patients with CNCP, when they come to attend training sessions for general practice teams offered monthly at Witten/Herdecke University. Additionally, an invitation letter will be distributed via email to practices associated with the Institute of General Practice and Primary Care, Witten/Herdecke University, as teaching and/or research practices.
Patients with CNCP will be approached via teaching and research practices associated with the university Institute of General Practice and Primary Care and via patient representatives supporting the study in the GESCO advisory board.
For both GPs and patients, a balanced gender ratio will be taken into account. GPs and patients who participate in the interviews will also be asked to attend the workshop in order to support the development of the intervention.
Data collection methods and data management
Semi-structured interviews with GPs and patients will be conducted in person at Witten/Herdecke University, via telephone, or using a certified video conferencing system. The guidelines, which will be used for structuring the interviews, will address patients’ and GPs’ experiences regarding CNCP management, patients’ wishes related to CNCP care, and GPs’ needs for treating patients with CNCP (Supplementary Material 1 and 2). The interview guides will consider findings from the previous searches. The development will be supported by GP and patient representatives. All interviews will be conducted by researchers who are trained in qualitative methodologies. No individuals other than the interviewed person and the interviewer will be present.
In order to facilitate the analyses, all interviews will be audio-recorded and transcribed verbatim. All statements identifying a person will be anonymized in the transcription process.
Data analysis
In order to make the findings of the interviews promptly available for the development of the intervention, analyses of the qualitative data will be performed using rapid qualitative analysis [47]. The coding process will be responsibly managed by two researchers. The resulting themes of the qualitative analysis will be presented to stakeholders in workshops through structured reports and thematic maps. This information will guide discussions on refining and prioritizing elements of the intervention, ensuring it aligns with the qualitative insights from the study.
Phase II: pilot testing of the GESCO intervention and the implementation strategies
Study design
The pilot testing of the newly developed intervention and the strategies used for implementation will be conducted as a hybrid type 2 effectiveness-implementation study and follows the guidance for conducting feasibility and pilot studies for implementation trials according to Pearson et al. This design features simultaneous testing of both (a) the feasibility of implementation and (b) clinical parameters as co-primary aims [38, 48]. In detail, an exploratory, mixed-methods study consisting of the following elements will be conducted:
Mixed-methods process evaluation with predominantly qualitative methods in order to determine whether it is feasible to proceed to an effectiveness trial.
Patient-centered pre-post comparison to quantify preliminary interventional effects in order to get a preview of the magnitude the intervention might have and to prepare a subsequent effectiveness trial.
Study setting and eligibility criteria
GPs will be eligible for the feasibility study if they manage patients with CNCP in their everyday practice and prescribe opioids for CNCP treatment. Patients will be included if they are at least 18 years old, suffer from CNCP, and have been receiving opioid therapy for at least 3 months. Patients with a clinically relevant malignant primary disease, patients currently receiving medications for opioid use disorder, and those with insufficient German language skills for participation will be excluded. A balanced gender ratio will be considered for both GPs and patients with CNCP.
Sample size and recruitment
Ten GPs from different general practices will be recruited for the feasibility study, excluding those practices and GPs that already participated in the development process. Again, GPs will be informed about the study and invited to participate during training sessions at the university and via email distributed to the institutes’ teaching and research practice network. Considering a patient drop-out rate of 20% and a target sample size of 40 patients for the analysis, each of the ten GPs will recruit five patients fulfilling the eligibility criteria defined for the study. This sample size is sufficient to detect, e.g., a mean difference (before and after intervention) of 0.5 on the 10-point-pain scale with a standard deviation of 1 (alpha = 0.05, power = 80%, 2-sided one-sample-t-test).
In order to ensure that the patients recruited for the feasibility study will match the eligibility criteria, study team will provide the GPs comprehensive written information and guidelines on how to conduct the feasibility study. To assess fidelity to the protocol, the study team will conduct study monitoring by periodic checks and also provide ongoing support to the GPs, ensuring they understand and adhere to the established procedures. This monitoring will help maintain consistency and compliance throughout the study.
Implementation
In order to implement the GESCO intervention in the care of patients with CNCP, the following implementation strategies [49] will be applied:
- Educational training for GPs: Based on current knowledge GPs will take part in two educational sessions addressing
- Pharmacotherapy (targeted use of assessments, i.e., in order to screen for opioid use disorder; sex-specific pharmacotherapy; drug therapy safety)
- Strategies for patient empowerment (concepts for “de-chronification” in CNCP in consideration of the pain medications’ role for patients and their significance for coping with everyday life)
- Communication training (narrative interview techniques to facilitate a sex-, gender-, and diversity-sensitive exploration of patients; health-oriented conversation in order to promote patients’ health competencies and salutogenesis)
- Self-reflection (reflection of the GPs’ own medical actions in consideration of sex- and gender-sensitive aspects; reflection of the GPs’ individual gender role and gender awareness; introduction of mind–body approaches, i.e., stress management approaches, including practical exercises on how to instruct patients).
Case conferences: online quality circles for GPs to discuss care management of patients with CNCP.
Support: Materials and infrastructure facilitating the sustainability of the intervention in daily routine, i.e., handouts regarding communication strategies and a pharmacological hotline.
The training program will be developed in accordance with the principles for resilient learning programs of Haraldseid-Driftland and colleagues [50].
Intervention
The GESCO intervention comprises a sex- and gender-sensitive care concept for patients with CNCP in primary care. Applying knowledge and skills from the previous educational training, GPs will conduct two counseling sessions within 6 to 8 weeks with each of the study patients. During the counseling sessions, they are requested to obtain an expanded medical history also considering sex and gender aspects, perform a medication analysis, provide resource-oriented counseling, and—if necessary—refer the patients for psychosocial support.
The final choice of which intervention content to apply will be left to the GP’s discretion depending on the patient’s needs.
Outcomes
With the overall goal of determining whether a larger effectiveness trial is appropriate, this study will simultaneously test (a) the feasibility of implementing the intervention and of the trial methods and (b) the intervention’s clinical outcomes [38]:
Implementation measures will be assessed to obtain detailed information on the implementation process and its feasibility (Table 1).
Patient-centered clinical outcomes will be assessed to evaluate the instruments’ appropriateness and to determine preliminary effects, which will—also considering clinical relevance and results from other studies—provide the basis for an effect size estimation for a future larger effectiveness trial (Table 2).
Table 1.
Feasibility measures based on Pearson et al. [38]
| Measure | Criteria | Data collection | Date of data collection |
|---|---|---|---|
| Implementation measures addressing the recruitment process | |||
| Reach | Recruitment of 10 practices for the study successful within 3 months | Documentation sheet, completed by study center | Continuously during practice recruitment and study conduct |
| Success of different recruitment strategies applied for GP recruitment | |||
| Drop outs (GPs and patients) | |||
| Recruitment of 50 study patients by 10 practices successful within 3 months | Documentation sheet, completed by the practices | Continuously during patient recruitment | |
| Patients willingness to receive the GESCO intervention/participate in the study | Qualitative interview with GPs | After completing two consultations per study patient (t1) | |
| Implementation measures addressing the educational training for GPs | |||
| Adoption | Uptake (participation in educational trainings) | Documentation sheet, completed by study center | After educational training |
| Acceptability | If GPs find the intervention’s components agreeable | Evaluation sheet, completed by GPs | After educational training |
| Self-efficacy | Self-perceived capacity to undertake implementation | ||
| Implementation measures addressing the intervention on patient level | |||
| Fidelity | Degree to which interventional components are implemented as intended by designers (adherence) | Documentation sheet for each consultation with study patient, completed by GP | After each patient contact |
| Feasibility | Perceived fit of the intervention for everyday use | Qualitative interview with GPs | After completing two consultations per study patient (t1) |
| Adaptability | Adaptability of the intervention’s components to meet local needs | ||
| Satisfaction | Satisfaction with the implementation strategies and intervention | ||
| Sustainability of implementation | |||
| Sustainability | Uptake of intervention | Qualitative interview with GPs incl. the G-NoMAD [53] | After completing two consultations per study patient (t1) |
| Written questionnaire incl. the G-NoMAD [53], completed by GP | Simultaneously with last patient-out per practice | ||
Table 2.
Patient-centered clinical outcomes
| Outcome | Measure | Data collection | Date of data collection | ||
|---|---|---|---|---|---|
| t0 | t1 | t2 | |||
| Pain | Pain history and progression (German Pain Questionnaire [58]) | Self-assessment, completed by patient | x | x | |
| 10-point-pain scale (three scales: acute pain, average pain during the last 4 weeks, and strongest pain within the last 4 weeks) | Telephone interview with patient, conducted by study nurse | x | x | x | |
| Mental well-being | Depression-Anxiety-Stress Scale (DASS) [59] | Self-assessment, completed by patient | x | x | |
| The Marburg questionnaire on habitual well-being (FW7) [60] | |||||
| Quality of life | Veterans RAND 12-Item Health Survey (VR-12) [61] | ||||
| Pain medication | Self-reported medication | Telephone interview with patient, conducted by study nurse | x | x | x |
| German, nationally standardized medication plan | Assessed by treating physician | x | x | x | |
| Adverse effects of medication | Self-reported adverse effects | Telephone interview with patient, conducted by study nurse | x | x | |
| Satisfaction with information about medication | Satisfaction with Information about Medicines Scale (SIMS) [62, 63] | ||||
| Potential opioid medication misuse | Pain Medication Questionnaire (PMQ) [64] | ||||
| Perceived stigma due to pain | Internalized Stigma of Chronic Pain (ISCP) [65]a | ||||
| Disruption of daily life due to pain | Pain Disability Index (PDI) [66] | ||||
| Optimism/pessimism | Optimism–Pessimism Short Scale 2 (SOP2) [67] | ||||
t0 baseline (before intervention); t1 2-month follow-up; t2 5-month follow-up
aGerman translation by study team
Data collection and data management
Data from the participating GPs will be collected immediately after recruitment, after participation in the training, during implementation of the GESCO intervention in patient care, and after completing the intervention for all study patients (Table 1).
After recruitment, GPs will complete a written questionnaire on practice characteristics, sociodemographic characteristics, and gender awareness (Nijmegen Gender Awareness in Medicine Scale N-GAMS [50, 51]).
After participation in the educational sessions, GPs will provide written feedback regarding the sessions’ content, the materials used, the acceptability of the intervention within their practice, and their self-efficacy to apply the intervention in patient care. Also, the practices’ Organizational Readiness for Implementing Change will be assessed using the validated ORIC questionnaire [51, 52].
When implementing the intervention in CNCP care, GPs will complete a documentation sheet for each patient consultation which assesses the duration, main contents and results of the consultation, the interventional elements applied, and the GPs personal impression of the consultation. These quantitative data will be manually entered into an electronic data capture system.
For data collections with GPs that will be conducted after completing the intervention for all study patients, qualitative and quantitative methods will be applied. Immediately after finishing two consultations per study patient, a semi-structured telephone interview will be conducted with each GP (Supplementary Material 3). These interviews will be audio-recorded and transcribed afterwards. During transcription, data will be pseudonymized using a unique identification number per GP in order to link the interview data to the quantitative data assessed per physician. Accompanying the interview, the Normalization Process Theory Measure (G-NoMAD) [53, 54] will be obtained to assess the implementation process. Simultaneously with the last patient-out per practice, each GP will complete a questionnaire on sustainability aspects, which will again include the G-NoMAD [53].
In addition to the feasibility aspects assessed from the GPs, aspects on the methods’ feasibility related to the recruitment process and the implementation will be documented by the study team in order to prepare a future larger effectiveness trial (Table 1).
For patients, data will be collected three times: after enrollment (baseline, t0), about 2 months later (immediately after completing the intervention, t1), and 5 months after baseline (t2) (Table 2). All data for the patient-centered pre-post comparison, including self-reported prescribed and over-the-counter medication, will be collected from the patients via phone at baseline and after 5 months. To describe the study population sociodemographic data, gender-related variables for health research (GVHR [55], German translation by study group), and experience of social support (Oslo Social Support Scale OSSS-3 [56, 57]) will be collected at baseline. This procedure will be facilitated by a study nurse who will enter the data directly into an electronic data capture system. The data collection at 2 months will consist of a qualitative, semi-structured telephone interview and will focus on feasibility outcomes (Supplementary Material 4). In detail, the open-ended questions will address the patients’ experience with the intervention and their study participation. This includes their satisfaction with the intervention, their acceptability of the intervention, especially regarding the gender approach, and how they perceived the communication with their GP, with the study center and the data collection process. In addition to qualitative, open-ended questions, any changes in medication use since baseline will be assessed. The interview part on patients’ experiences will be audio-recorded and transcribed verbatim, whereas the data on medications will be entered directly into the electronic data capture system. Qualitative interview data will be pseudonymized using a unique identification number per patient in order to link the interview data to the quantitative data assessed for the pre-post comparison. Additionally, GPs will complete a documentation sheet for each participating patient at baseline and after 5 months, providing information on chronic diseases and prescribed medication. Medication use will be analyzed after Anatomical Therapeutic Chemical coding and in consideration of drug dosages and frequency of administration.
Data analysis
Analyses of the qualitative data will be performed in MAXQDA 2022 [68] using a deductive-inductive approach. To this end, deductive categories will first be defined on the basis of the interview guides. Afterwards, the coding schemes will be continuously developed and refined over time by identifying categories directly from the text material. The coding process will be responsibly managed by two researchers and will include coding sessions with a group of researchers from the GESCO study team, GP representatives, and patient representatives with CNCP.
For quantitative data, descriptive statistics will be performed using IBM SPSS Statistics for Windows [69] in order to measure preliminary effects. Patients’ baseline and follow-up data will be compared by applying a t-test for dependent samples, the Wilcoxon test or McNemar’s/the sign test depending on the distribution of the outcome variable. The nominal significance level for analyses will be defined as p < 0.05. In addition, confidence intervals will be reported for any quantities estimated.
Patient and public involvement
The realization of the GESCO study is accompanied by a multi-perspective advisory board that includes female and male patient representatives, but also experts in general practice, pain medicine, addiction medicine, psychology, health care education, sociology, gender research, and participatory research. The advisory board members will be informed about the study process and asked for advice in regular meetings. In addition to these meetings, they will get actively involved into the following activities:
Development of the intervention and its implementation strategy within primary care
Preparation of the feasibility study including the development and pre-testing of study material for patients and GPs, interview guidelines, and questionnaire
Recruitment of interview partners for the needs assessments and recruitment of primary care practices for the feasibility study
The discussion and dissemination of results including contribution to conference presentations and to scientific or low-threshold, generally understandable publications (e.g., flyers or brochures)
In order to adequately consider the perspective of potential addressees of the GESCO intervention during the whole study, researchers working in the project planned and reflected involvement activities, which especially affect patient representatives and GPs, together with these stakeholders. For this, they used the so-called involvement matrix [70]. This process was already carried out prior to the beginning of phase I and was facilitated by an advisory board member familiar with applying the involvement matrix. It aimed to enable everyone to specify how intensively and in which project phases they would like to get involved or not to get involved.
Ethics and dissemination
Ethics approval
The study obtained ethical approval from the Ethics Commission of Witten/Herdecke University (reference number: 138/2022, date of approval: 08/25/2022, amendment: 08/29/2023).
Dissemination policy
As is customary, it is planned to publish a description of the intervention components and the results of the pilot testing in international journals and to present all results at scientific conferences. In order to also make the study results transparent and comprehensible for the non-scientific public, a GESCO symposium addressing GPs, patients, researchers, and the public will be conducted at the end of the project. For this, the study conduct and its results will be prepared in simple language, which will be facilitated by patient representatives, GPs, and other members of the GESCO advisory board. Beside the public symposium, it is planned to disseminate the results at a low threshold level, i.e., via magazines of self-help organizations. The dissemination strategy will be planned together with patient representatives and GPs.
The dissemination policy will also include an analysis of whether the sex- and gender-sensitive concept developed for CNCP management might be transferable to other health care scenarios.
As the project is part of a larger funding initiative of the German Federal Ministry of Health, which aims to investigate and establish gender equality in health, information on the GESCO project and its results will also be published on the ministry’s website.
Discussion
The GESCO study will examine how the diversity of the experience and processing of pain in patients on LTOT of differing sex and gender can be addressed appropriately and in a quality-enhancing manner in the therapeutic setting. As a result, it will provide a novel personalized concept for the care of patients with CNCP, integrated into preliminary analyses and a subsequent feasibility assessment to ascertain the suitability and implementability of the intervention. It will also be used to pilot the study instruments and measures. Our results regarding the implementation measures will be evaluated to determine the suitability of the intervention for transfer to an efficacy trial. If necessary, the intervention will be adapted or (in the worst case) rejected. The progression criteria, as outlined by Thabane and Lancaster [41] and suggested by Pearson et al. [38], will be used to guide this assessment. The limited sample size of the GESCO study restricts the generalizability of possible interventional effects but the results of this study build a foundation to estimate the sample size for a subsequent cluster randomized controlled trial taking into account the standard deviation. Additionally our estimation will be complemented by other studies from the literature applying the same outcome measures.
By considering sex and gender differences in health care and proposing a sex- and gender-sensitive care concept, the GESCO study team is doing pioneering work, which might also be transferable to other health care scenarios and thereby help improve disease management.
Supplementary Information
Acknowledgements
Our special thank goes to the members of our GESCO advisory board. Moreover, we thank all GPs and patients who support our study.
Consortia on behalf of the GESCO study group
Achim Mortsiefer, Christine Kersting, Johannes Just, Klaus Weckbecker, Alexandra Schmidt, Alexandra Piotrowski, Michaela Duck, Neele Kufeld, Rebecca Bisplinghoff and Michaela Maas (Institute of General Practice and Primary Care, Witten/Herdecke University), Petra Thürmann and Sven Schmiedl (Chair of Clinical Pharmacology, Witten/Herdecke University; Philipp Klee-Institute of Clinical Pharmacology, Helios University Hospital Wuppertal), Veronika Bencheva and Jordan Preuß (Chair of Clinical Pharmacology, Witten/Herdecke University), Birgitt Wiese (IT Services Applications, Science & Laboratory, MHH Information Technology, Hannover Medical School), and all members of the advisory board, namely: Ottomar Bahrs (Institute of General Practice, Heinrich-Heine-University Düsseldorf; Umbrella Organization Salutogenesis, Göttingen), Thomas Cegla (Department of Pain Medicine, Helios Universitätsklinikum Wuppertal), Sigrid Elsenbruch and Claudia Levenig (Department of Medical Psychology and Medical Sociology, Ruhr University Bochum), Christina Hunger-Schoppe (Department of Psychology and Psychotherapy, Witten/Herdecke University), Claudia Kiessling (Chair of Personal and Interpersonal Development in Health Care Education, Witten Herdecke/University), Ulrich Klee, Ursula Lauf and Brigitte Scholz (patient representatives), Albine Moser (CAPHRI Institute, Department of Family Medicine, Maastricht University), Norbert Scherbaum and Michael Specka (Department of Addictive Behavior and Addiction Medicine, LVR-Hospital Essen, University of Duisburg-Essen).
Abbreviations
- CNCP
Chronic non-cancer pain
- DASS
Depression-Anxiety-Stress Scale
- FW7
The Marburg questionnaire on habitual well-being
- GESCO
Gender-sensitive care for chronic non-cancer pain patients receiving long-term opioid therapy
- GVHR
Gender-related variables for health research
- GP
General practitioner
- ISCP
Internalized Stigma of Chronic Pain
- LTOT
Long-term opioid therapy
- MRC
UK Medical Research Council
- N-GAMS
Nijmegen Gender Awareness in Medicine Scale
- NoMAD
Normalization Process Theory Measure
- ORIC
Organizational Readiness for Implementing Change
- OSSS-3
Oslo Social Support Scale
- PDI
Pain Disability Index
- PMQ
Pain Medication Questionnaire
- SIMS
Satisfaction with Information about Medicines Scale
- SOP2
Optimism-Pessimism Short Scale-2
- VR-12
Veterans RAND 12-Item Health Survey
Authors' contributions
AM and KW had the initial idea for the project. AM, CK, JJ, KW, PT, and BW initiated the study design, conceptualized the study including the measurement methods, and submitted an application for external funding. BW provided statistical expertise; PT provided pharmacological expertise. All authors contributed to the development of the study materials needed. AS, CK, AP, and AM created and submitted the ethics proposal and drafted the manuscript, which was critically reviewed by all other authors. All authors and members of the GESCO study group approved the final manuscript and agreed to publication.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is funded by the German Federal Ministry of Health, funding numbers 2522FSB14A and 2522FSB14B. The sponsor did not play any role in the study design and conceptualization or in writing this manuscript.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
The GESCO study will be performed in accordance with the Declaration of Helsinki. It obtained ethical approval from the Ethics Commission of Witten/Herdecke University (reference number: 138/2022, date of approval: 08/25/2022; amendment: 08/29/2023). All GPs and patients who participate in the study during the development process or during pilot testing will receive written information and provide informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christine Kersting, Email: Christine.Kersting@uni-wh.de.
on behalf of the GESCO study group:
Christine Kersting, Johannes Just, Alexandra Piotrowski, Alexandra Schmidt, Neele Kufeld, Rebecca Bisplinghoff, Michaela Maas, Veronika Bencheva, Jordan Preuß, Birgitt Wiese, Klaus Weckbecker, Achim Mortsiefer, Petra Thürmann, Michaela Duck, Sven Schmiedl, Ottomar Bahrs, Thomas Cegla, Sigrid Elsenbruch, Claudia Levenig, Christina Hunger-Schoppe, Claudia Kiessling, Ulrich Klee, Ursula Lauf, Brigitte Scholz, Albine Moser, Norbert Scherbaum, and Michael Specka
References
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plass D, Vos T, Hornberg C, Scheidt-Nave C, Zeeb H, Krämer A. Trends in disease burden in Germany: results, implications and limitations of the Global Burden of Disease study. Dtsch Arztebl Int. 2014;111:629–38. 10.3238/arztebl.2014.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Gureje O, von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280:147–51. 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother. 2013;13:1201–20. 10.1586/14737175.2013.846517. [DOI] [PubMed] [Google Scholar]
- 6.Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep. 2017;2:e599. 10.1097/PR9.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bletzer J, Gantz S, Voigt T, Neubauer E, Schiltenwolf M. Chronische untere Rückenschmerzen und psychische Komorbidität : Eine Übersicht. [Chronic low back pain and psychological comorbidity: a review]. Schmerz. 2017;31:93–101. 10.1007/s00482-016-0143-4. [DOI] [PubMed] [Google Scholar]
- 8.Voon P, Karamouzian M, Kerr T. Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy. 2017;12:36. 10.1186/s13011-017-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Just J, Petzke F, Scherbaum N, Radbruch L, Weckbecker K, Häuser W. Kritische Auseinandersetzung mit neuen Daten zur Prävalenz von Opioidgebrauchsstörungen bei Patienten mit chronischen Schmerzen in Deutschland. [Critical discussion of new data regarding prevalence of opioid use disorder in patients with chronic pain in Germany]. Schmerz. 2022;36:13–8. 10.1007/s00482-021-00582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roter DL, Hall JA. Physician gender and patient-centered communication: a critical review of empirical research. Annu Rev Public Health. 2004;25:497–519. 10.1146/annurev.publhealth.25.101802.123134. [DOI] [PubMed] [Google Scholar]
- 11.Marschall U, L’hoest H, Radbruch L, Häuser W. Long-term opioid therapy for chronic non-cancer pain in Germany. Eur J Pain. 2016;20:767–76. 10.1002/ejp.802. [DOI] [PubMed] [Google Scholar]
- 12.Just JM, Scherbaum N, Specka M, Puth M-T, Weckbecker K. Rate of opioid use disorder in adults who received prescription opioid pain therapy-a secondary data analysis. PLoS ONE. 2020;15:e0236268. 10.1371/journal.pone.0236268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 14.Munce SEP, Stewart DE. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics. 2007;48:394–9. 10.1176/appi.psy.48.5.394. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll MA, Higgins DM, Seng EK, Buta E, Goulet JL, Heapy AA, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16:1101–11. 10.1111/pme.12744. [DOI] [PubMed] [Google Scholar]
- 16.Regitz-Zagrosek V, Seeland U. Sex and gender differences in clinical medicine. Handb Exp Pharmacol. 2012:3–22. 10.1007/978-3-642-30726-3_1. [DOI] [PubMed]
- 17.Gazerani P, Aloisi AM, Ueda H. Editorial: differences in pain biology, perception, and coping strategies: towards sex and gender specific treatments. Front Neurosci. 2021;15:697285. 10.3389/fnins.2021.697285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne NR, Davis KD. Sex and gender differences in pain. Int Rev Neurobiol. 2022;164:277–307. 10.1016/bs.irn.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res. 2017;95:1271–81. 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 20.Franconi F, Campesi I. Sex and gender influences on pharmacological response: an overview. Expert Rev Clin Pharmacol. 2014;7:469–85. 10.1586/17512433.2014.922866. [DOI] [PubMed] [Google Scholar]
- 21.Oi Yan Chan J, Moullet M, Williamson B, Arends RH, Pilla Reddy V. Harnessing clinical trial and real-world data towards an understanding of sex effects on drug pharmacokinetics, pharmacodynamics and efficacy. Front Pharmacol. 2022;13:874606. 10.3389/fphar.2022.874606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: modulatory role of gonadal hormones. Life Sci. 2019;237:116926. 10.1016/j.lfs.2019.116926. [DOI] [PubMed] [Google Scholar]
- 23.Pisanu C, Franconi F, Gessa GL, Mameli S, Pisanu GM, Campesi I, et al. Sex differences in the response to opioids for pain relief: a systematic review and meta-analysis. Pharmacol Res. 2019;148:104447. 10.1016/j.phrs.2019.104447. [DOI] [PubMed] [Google Scholar]
- 24.Riley JL, Robinson ME, Wade JB, Myers CD, Price DD. Sex differences in negative emotional responses to chronic pain. J Pain. 2001;2:354–9. 10.1054/jpai.2001.27000. [DOI] [PubMed] [Google Scholar]
- 25.Werner A, Malterud K. It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Soc Sci Med. 2003;57:1409–19. 10.1016/s0277-9536(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 26.Keogh E. Gender differences in the nonverbal communication of pain: a new direction for sex, gender, and pain research? Pain. 2014;155:1927–31. 10.1016/j.pain.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Bernardes SF, Lima ML. Being less of a man or less of a woman: perceptions of chronic pain patients’ gender identities. Eur J Pain. 2010;14:194–9. 10.1016/j.ejpain.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Miron-Shatz T, Ormianer M, Rabinowitz J, Hanoch Y, Tsafrir A. Physician experience is associated with greater underestimation of patient pain. Patient Educ Couns. 2020;103:405–9. 10.1016/j.pec.2019.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Denberg TD, Barry MJ, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–30. 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 30.Häuser W, Klose P, Welsch P, Petzke F, Nothacker M. Leitlinienreport der zweiten Aktualisierung der S3-Leitlinie „Langzeitanwendung von Opioiden bei nicht-tumorbedingten Schmerzen – LONTS“. [Method report of the second update of the guidelines on long-term opioid therapy for chronic noncancer pain]. Schmerz. 2020;34:245–78. 10.1007/s00482-020-00471-z. [DOI] [PubMed] [Google Scholar]
- 31.Smith HAB, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A, et al. 2022 Society of Critical Care Medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med. 2022;23:e74–110. 10.1097/PCC.0000000000002873. [DOI] [PubMed] [Google Scholar]
- 32.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–45. 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dielissen P, Bottema B, Verdonk P, Lagro-Janssen T. Attention to gender in communication skills assessment instruments in medical education: a review. Med Educ. 2011;45:239–48. 10.1111/j.1365-2923.2010.03876.x. [DOI] [PubMed] [Google Scholar]
- 34.Burt J, Lloyd C, Campbell J, Roland M, Abel G. Variations in GP-patient communication by ethnicity, age, and gender: evidence from a national primary care patient survey. Br J Gen Pract. 2016;66:e47-52. 10.3399/bjgp15X687637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandhu H, Adams A, Singleton L, Clark-Carter D, Kidd J. The impact of gender dyads on doctor-patient communication: a systematic review. Patient Educ Couns. 2009;76:348–55. 10.1016/j.pec.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Dielissen P, Verdonk P, Bottema B, Kramer A, Lagro-Janssen T. Expert consensus on gender criteria for assessment in medical communication education. Patient Educ Couns. 2012;88:189–95. 10.1016/j.pec.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, Bond CM. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE. 2016;11:e0150205. 10.1371/journal.pone.0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson N, Naylor P-J, Ashe MC, Fernandez M, Yoong SL, Wolfenden L. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020;6:167. 10.1186/s40814-020-00634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, Tyrer P. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321:694–6. 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. 10.1136/bmj.n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thabane L, Lancaster G. A guide to the reporting of protocols of pilot and feasibility trials. Pilot Feasibility Stud. 2019;5:37. 10.1186/s40814-019-0423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilissen J, Pivodic L, Gastmans C, Vander Stichele R, Deliens L, Breuer E, van den Block L. How to achieve the desired outcomes of advance care planning in nursing homes: a theory of change. BMC Geriatr. 2018;18:47. 10.1186/s12877-018-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson AA. The community builder’s approach to theory of change: a practical guide to theory development. The Aspen Institute Roundtable on Community Change; 2009. https://www.theoryofchange.org/pdf/TOC_fac_guide.pdf.
- 46.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26:1753–60. 10.1177/1049732315617444. [DOI] [PubMed] [Google Scholar]
- 47.Nevedal AL, Reardon CM, Opra Widerquist MA, Jackson GL, Cutrona SL, White BS, Damschroder LJ. Rapid versus traditional qualitative analysis using the Consolidated Framework for Implementation Research (CFIR). Implement Sci. 2021;16:67. 10.1186/s13012-021-01111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–26. 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haraldseid-Driftland C, Lyng HB, Guise V, Waehle HV, Schibevaag L, Ree E, et al. Learning does not just happen: establishing learning principles for tools to translate resilience into practice, based on a participatory approach. BMC Health Serv Res. 2023;23:646. 10.1186/s12913-023-09653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. 2014;9:7. 10.1186/1748-5908-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindig A, Hahlweg P, Christalle E, Scholl I. Translation and psychometric evaluation of the German version of the Organisational Readiness for Implementing Change measure (ORIC): a cross-sectional study. BMJ Open. 2020;10:e034380. 10.1136/bmjopen-2019-034380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freund J, Piotrowski A, Bührmann L, Oehler C, Titzler I, Netter A-L, et al. Validation of the German Normalization Process Theory Measure G-NoMAD: translation, adaptation, and pilot testing; 2023. [DOI] [PMC free article] [PubMed]
- 54.Finch TL, Girling M, May CR, Mair FS, Murray E, Treweek S, et al. NoMAD: Implementation measure based on Normalization Process Theory. [Measurement instrument]. 2015. Retrieved from http://www.normalizationprocess.org.
- 55.Nielsen MW, Stefanick ML, Peragine D, Neilands TB, Ioannidis JPA, Pilote L, et al. Gender-related variables for health research. Biol Sex Differ. 2021;12:23. 10.1186/s13293-021-00366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalgard OS, Bjørk S, Tambs K. Social support, negative life events and mental health. Br J Psychiatry. 1995;166:29–34. 10.1192/bjp.166.1.29. [DOI] [PubMed] [Google Scholar]
- 57.Kocalevent R-D, Berg L, Beutel ME, Hinz A, Zenger M, Härter M, et al. Social support in the general population: standardization of the Oslo social support scale (OSSS-3). BMC Psychol. 2018;6:31. 10.1186/s40359-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petzke F, Hüppe M, Kohlmann T, Kükenshöner S, Lindena G, Pfingsten M, et al. Handbuch Deutscher Schmerz-Fragebogen. 2022. https://www.schmerzgesellschaft.de/fileadmin/2022/PDFs/DSF_Handbuch_2022.pdf.
- 59.Nilges P, Köster B, Schmidt CO. Schmerzakzeptanz - Konzept und Uberprüfung einer deutschen Fassung des chronic pain acceptance questionnaire. [Pain acceptance - concept and validation of a German version of the chronic pain acceptance questionnaire]. Schmerz. 2007;21(57–8):60–7. 10.1007/s00482-006-0508-1. [DOI] [PubMed] [Google Scholar]
- 60.Basler HD. Marburger Fragebogen zum habituellen Wohlbefinden - Untersuchung an Patienten mit chronischem Schmerz. Schmerz. 1999;13:385–91. [DOI] [PubMed] [Google Scholar]
- 61.Buchholz I, Feng Y-S, Buchholz M, Kazis LE, Kohlmann T. Translation and adaptation of the German version of the Veterans Rand-36/12 Item Health Survey. Health Qual Life Outcomes. 2021;19:137. 10.1186/s12955-021-01722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care. 2001;10:135–40. 10.1136/qhc.0100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahler C, Jank S, Hermann K, Horne R, Ludt S, Haefeli WE, Szecsenyi J. Psychometric properties of a German version of the “Satisfaction with Information about Medicines Scale” (SIMS-D). Value Health. 2009;12:1176–9. 10.1111/j.1524-4733.2009.00575.x. [DOI] [PubMed] [Google Scholar]
- 64.Adams LL, Gatchel RJ, Robinson RC, Polatin P, Gajraj N, Deschner M, Noe C. Development of a self-report screening instrument for assessing potential opioid medication misuse in chronic pain patients. J Pain Symptom Manage. 2004;27:440–59. 10.1016/j.jpainsymman.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. J Pain. 2014;15(550):e1-10. 10.1016/j.jpain.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Dillmann U, Nilges P, Saile H, Gerbershagen HU. PDI - Pain Disability Index - deutsche Fassung: ZPID (Leibniz Institute for Psychology) – Open Test Archive; 2011.
- 67.Kemper CJ, Wassermann M, Hoppe A, Beierlein C, Rammstedt B. Measuring dispositional optimism in large-scale studies: psychometric evidence for German, Spanish, and Italian versions of the Scale Optimism-Pessimism-2 (SOP2). Eur J Psychol Assess. 2017;33:403–8. 10.1027/1015-5759/a000297. [Google Scholar]
- 68.VERBI Software. MAXQDA. Berlin, Germany: VERBI Software; 2022.
- 69.IBM Corp. IBM SPSS Statistics for Windows. Armonk, New York: IBM Corp; 2021.
- 70.Smits D-W, van Meeteren K, Klem M, Alsem M, Ketelaar M. Designing a tool to support patient and public involvement in research projects: the involvement matrix. Res Involv Engagem. 2020;6:30. 10.1186/s40900-020-00188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.