Abstract
Background
This study aimed to clarify the effectiveness of nutrition support team (NST) facilities for preventing central line-associated bloodstream infection (CLABSI).
Methods
We retrospectively analyzed the incidence of CLABSI as well as the presence or absence of additional medical fees for NST activity between 2019 and 2021, including the period before and after the COVID-19 pandemic. Subsequently, we performed between-group comparisons of the CLABSI incidence. CLABSI rates were compared based on cumulative per 1000 catheter uses during the relevant period.
Results
Among 47 facilities that were registered for participation, there were 34 and 13 facilities with and without additional medical fees for NST activity (NST and non-NST groups, respectively). The CLABSI incidence rate was significantly lower in the NST group 0.96 [0.28–1.73] than in the non-NST group 1.25 [075–6.10] (p < 0.05). Before the pandemic, the NST group had a lower CLABSI rate per 1000 catheter uses than the non-NST group 2019: 0.70 [0.12–1.26] vs 2.10 [0.62–5.97]. During the pandemic, the CLABSI incidence showed no significant between-group difference 2020: 0.99 [0.51–1.61] vs 1.01 [0.80–4.16]; 2021: 1.24 [0.44–2.35] vs 1.96 [1.23–5.31]; however, the CLABSI rates in the NST group remained low.
Conclusion
During the COVID-19 pandemic, the incidence of CLABSI was lower in the NST group than in the non-NST group, indicating the effectiveness of NST in preventing the occurrence of CLABSI.
Keywords: Central line-associated bloodstream infection, Nutrition support team, COVID-19 pandemic, Additional medical fee for the nutrition support team activity, Pharmacy
Background
Catheter-related bloodstream infections (CRBSIs) are the most common healthcare-associated infections. Worldwide, there has been extensive research on central line-associated bloodstream infections (CLABSIs) specifically, as they have been associated with extended duration of hospital stay, high mortality rates, and increased medical costs [1–5]. In the United States, with the development and adaption of central parenteral nutrition, there has been a concomitant need to suppress related complications such as CLABSI; accordingly, the nutrition support team (NST) was established [6, 7]. In Japan, the NST has been popularized through the initiative of the Japan Society for Nutritional Therapy. Further, in the 2010 medical fee revision, NST facilities were defined as those with an additional medical fee for NST activity. The NST comprises specialized doctors, nurses, pharmacists, and registered dietitians who attend to patients with malnutrition, blood albumin levels ≤ 3.0 g/dL, and a need for enteral or parenteral nutrition. The NST holds meetings and conferences to promote recovery and prevent complications such as infectious diseases. In addition, it holds joint conferences and collaborates with infection control teams. However, within the > 10-year period following the establishment of the NST, there have been very few nationwide studies on the effects of the NST on the incidence of CLABSI and parenteral nutrition management. Moreover, although the COVID-19 pandemic has affected various functions within medical institutions [8–10], the effect of the NST on the incidence of CLABSI during the COVID-19 pandemic remains unclear. Accordingly, this study aimed to investigate the effectiveness of NST in preventing the occurrence of CLABSI before and during the COVID-19 pandemic.
Materials and methods
Study design and population
This retrospective observational study included hospitalized patients diagnosed with CLABSI between January 2019 and December 2021. The primary endpoint was the incidence of CLABSI per 1000 catheters used; additionally, we compared the incidence of CLABSI between the NST and non-NST groups. CLABSI rates were compared based on cumulative per 1000 catheter uses during the relevant period.
The facility standard for infection control prevention additional medical fee 1 (ICP-1) is to have a full-time in-hospital infection control staff and an infection prevention department. An infection prevention team consisting of full-time doctors, nurses, pharmacists, and laboratory technicians is organized to carry out daily infection prevention work. The facility standard for infection control prevention additional medical fee 2 (ICP-2) is a facility with fewer than 300 general hospital beds. An infection prevention team consisting of full-time doctors or nurses is organized.
Data collection
We invited pharmacists who were members of the Japanese Society for Parenteral and Enteral Nutrition Therapy to participate in the study through the society's official website and direct mail. Data were collected using an electronic data collection system. The survey items included the type of medical facility, number of beds, number of patients with CLABSI, duration (days) of central venous catheter placement, ICP-1 and 2, CRBSI prevention manual and its documentation, sterile preparation processing fee, sterile preparation practices for total and peripheral parenteral nutrition, use of closed-system infusion lines and in-line filters, selection of disinfectants for central venous catheter insertion sites, and saline flush volume following fat emulsion administration.
The CRBSI prevention manual includes hand hygiene, indications and selection of catheter use, appropriate insertion method, in-line filters, infusion management method, CRBSI risk, and education for staff [11]. Among these, the principles for high-calorie infusion include choosing enteral nutrition whenever possible and mixing drugs into high-calorie infusion preparations in a sterile environment in the pharmacy whenever possible. Catheters are classified into short-term placement, long-term placement (Broviac catheter, Hickman catheter), completely subcutaneously implanted, and PICC (Peripherally inserted central venous catheter), and are selected according to the purpose of use. When inserting a central venous catheter, it is recommended that the insertion site be disinfected with chlorhexidine containing alcohol at a concentration of more than 0.5% and that maximal barrier precautions be used. In addition, since lipid emulsions and blood products have been identified as independent risk factors for CRBSI, the manual includes regular replacement of infusion sets and flushing methods for infusion lines.
Statistical analysis
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) as a graphical user interface for R version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria). EZR, a modified version of R Commander (version 1.61), is designed to incorporate commonly used statistical functions in biostatistics [12]. Continuous variables were expressed as medians and ranges, and categorical variables were summarized as frequencies and proportions. Between-group comparisons of quantitative and qualitative data were performed using the Mann–Whitney test, respectively. Statistical significance was set at p < 0.05.
Results
Facility information
There were 47 registered facilities (33 general hospitals, 10 mixed healthcare facilities, 3 nursing facilities, and 1 psychiatric hospital); among them, 34 and 13 facilities were in the NST and non-NST groups, respectively. Additionally, 85.3% and 53.8% of facilities in the NST and non-NST groups were general hospitals and mixed healthcare facilities, respectively. Regarding the number of beds, the NST group included large hospitals with > 600 beds while 30.8% of facilities in the non-NST group had < 200 beds. Further, 79.4% and 46.2% of facilities in the NST and non-NST groups, respectively, met the facility standards for ICP-1. Table 1 shows other facility information related to CLABSI.
Table 1.
Facility information
| Total n = 47 | NST group n = 34 | Non-NST group n = 13 | |
|---|---|---|---|
| National hospital | 2 (4.3) | 1 (3.0) | 1 (7.7) |
| Public hospital | 18 (38.3) | 15 (44.1) | 3 (23.1) |
| Private medical institutions | 27 (57.4) | 18 (52.9) | 9 (69.2) |
| General hospitals | 33 (70.2) | 29 (85.3)* | 4 (30.8) |
| Mixed healthcare facilities | 10 (21.3) | 3 (8.8) | 7 (53.8) |
| Nursing facilities | 3 (6.4) | 2 (5.9) | 1 (7.7) |
| Psychiatric hospital | 1 (2.1) | 0 (0.0) | 1 (7.7) |
| < 200 beds | 8 (17.0) | 4 (11.8) | 4 (30.8) |
| 200–599 beds | 30 (63.8) | 21 (64.7) | 9 (69.2) |
| > 600 beds | 9 (19.2) | 9 (26.5) | 0 (0.0) |
| Facilities for infection control prevention additional medical fee 1 | 33 (70.2) | 27 (79.4)** | 6 (46.2) |
| Facilities for infection control prevention additional medical fee 2 | 14 (29.8) | 7 (20.6) | 7 (53.8) |
| Presence of standards such as CRBSI prevention manuals | |||
| Standards ( +) / Documentation ( +) | 41 (87.2) | 31 (91.1) | 10 (76.9) |
| Standards ( +) / Documentation (-) | 5 (10.6) | 2 (5.8) | 3 (23.1) |
| Standards (-) / Documentation (-) | 1 (2.1) | 1 (2.9) | 0 (0.0) |
| Number of facilities with sterile preparation processing fee/non-fee (%) | 35/12 (74.5/25.5) | 27/7 (79.4/20.6) | 8/5 (61.5/38.5) |
| TPN sterile preparation rate | 80 [57–95] | 80 [70–90] | 83 [31–98] |
| PPN sterile preparation rate | 0 [0–5] | 0 [0–5] | 0 [0–38.7] |
| Number of closed-infusion systems used | 44 (93.6) | 31 (91.1) | 13 (100.0) |
| Number of inline filters used | 28 (59.5) | 18 (52.9) | 10 (76.9) |
| Disinfectant for CVC insertion site | |||
| Povidone–iodine | 33 (70.2) | 25 (73.5) | 8 (61.5) |
| Chlorhexidine–alcohol | 21 (44.6) | 17 (50.0) | 4 (30.7) |
| Ethanol | 8 (17.0) | 7 (20.5) | 1 (7.6) |
| Chlorhexidine | 6 (12.7) | 4 (11.7) | 2 (15.3) |
| Flush volume following fat emulsion administration | |||
| 5 mL saline | 5 (10.6) | 4 (11.7) | 1 (7.6) |
| 10 mL saline | 21 (44.6) | 14 (41.1) | 7 (53.8) |
| 20 mL saline | 15 (31.9) | 10 (29.4) | 5 (38.4) |
| 50 mL saline | 2 (4.2) | 2 (5.8) | 0 (0.0) |
% (number), Median [interquartile range]
*p < 0.01
**p < 0.05
Incidence of CLABSI
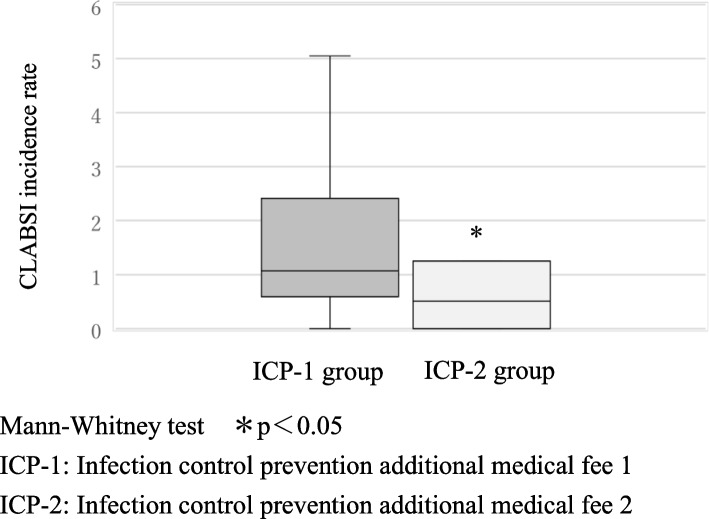
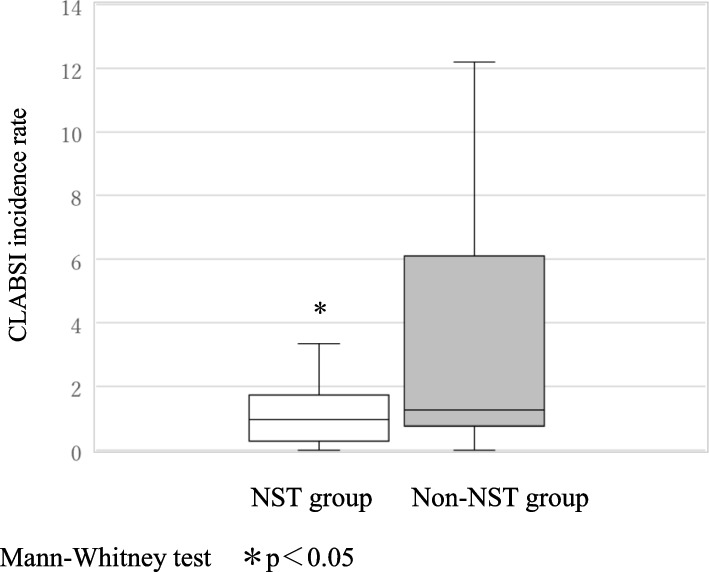
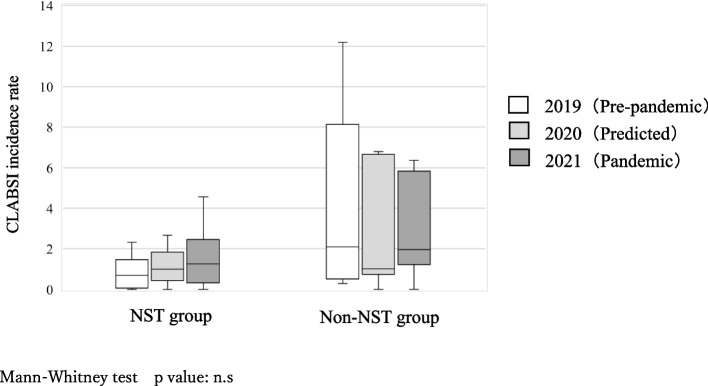
Over a 3-year period between 2019 and 2021, the CLABSI incidence rate per 1000 catheter uses was significantly lower in the ICP-2 group 0.50 [0.00—1.24] than in the ICP-1 group 1.07 [0.60—2.31], p < 0.05, (Fig. 1). Over a 3-year period between 2019 and 2021, the CLABSI incidence rate per 1000 catheter uses was significantly lower in the NST group 0.96 [0.28—1.73] than in the non-NST group 1.25 [0.75—6.10], p < 0.05, (Fig. 2). The overall CLABSI incidence per 1000 catheter uses was 0.70 [0.29—1.82] in 2019, 1.00 [0.52—1.73] in 2020, and 1.32 [0.55—2.59] in 2021. Before the pandemic, the NST group had a lower CLABSI rate per 1000 catheter uses than the non-NST group 2019: 0.70 [0.12—1.26] vs 2.10 [0.62—5.97]. During the pandemic, the CLABSI incidence showed no significant between-group difference 2020: 0.99 [0.51—1.61] vs 1.01 [0.80—4.16]; 2021: 1.24 [0.44—2.35] vs 1.96 [1.23—5.31]; however, the CLABSI rates in the NST group remained low (Fig. 3).
Fig. 1.
CLABSI incidence rate per 1000 catheter uses in ICP-1 and ICP-2 groups (Over a 3-year period from 2019 to 2021)
Fig. 2.
CLABSI incidence rate per 1000 catheter uses in NST and non-NST groups (Over a 3-year period from 2019 to 2021)
Fig. 3.
CLABSI incidence rate per 1000 catheter uses in NST and non-NST groups (before and during the pandemic)
Discussion
This study investigated the effectiveness of NST in preventing the occurrence of CLABSI before and during the COVID-19 pandemic.
Implementation of the NST has been associated with improved survival outcomes in critically ill patients with COVID-19 [13]. However, there have been no reports focusing on CLABSI and no studies on the NST in Japan. In Japan, the NST provides multi-disciplinary nutritional management to patients with malnutrition or those at risk of malnutrition to promote the healing of underlying diseases and to prevent complications such as infections. The training of healthcare professionals on infection prevention manuals for central venous catheter management is effective in reducing device-standardized infection rates and preventing CLABSI [14]. In the NST group, the documentation of the standards of the CLABSI prevention manual and the establishment of a system for implementing aseptic preparation could have contributed to the suppression of CLABSI occurrence. In our study, 93.6% and 59.5% of facilities used closed infusion systems and in-line filters, respectively, with no significant between-group difference. The use of antiseptics on central venous catheter insertion sites has demonstrated clinical preventive effects; among them, chlorhexidine–alcohol significantly reduces the incidence of CLABSI compared with povidone–iodine [15]. In addition, current guidelines recommend alcohol with chlorhexidine added at a concentration of > 0.5% [16]. Among the target facilities, povidone–iodine was the most widely used antiseptic. Chlorhexidine-induced anaphylaxis has been reported to be more common in the Japanese population than in the Caucasian population [17]. Accordingly, 10% povidone–iodine should be considered an option for skin disinfection; however, education on its usage is important. Fat emulsion administration is a risk factor for CLABSI [18]. Specifically, following fat emulsion administration, microorganisms that have entered the intravenous infusion line may proliferate over time. Therefore, it is important to flush the line with a sufficient saline volume to prevent CLABSI development. In both groups, the saline flush volume after fat emulsion administration was ≤ 10 mL in more than half of the patients. The required saline flush volume may be approximately twice the capacities of the indwelling needle, catheter, and connected device. Taken together, the administration of fat emulsions that promote microbial growth is an independent risk factor for CRBSI; therefore, it should be followed by an appropriate saline flush volume and frequent infusion set changes [19, 20].
During the COVID-19 pandemic, there was postponement or cancellation of elective surgeries as well as a need for an increased number of intensive care unit beds. Additionally, patients with COVID-19 have shown an increased incidence of various healthcare-associated infections, comorbidities, and long-term hospitalization [21, 22]. In our study, the incidence of CLABSI after the pandemic was non-significantly higher than that before the pandemic, which is consistent with previous findings [21, 22]. In 2020, the CLABSI incidence rate increased and decreased in the NST and non-NST groups, respectively. Before the pandemic, teamwork contributed to reducing the infection rate. During the pandemic, many facilities in the NST group were classified as Infection Control and Prevention Level 1; additionally, they received an increased number of patients since they accepted critically ill patients, which required targeted measures. Accordingly, during the pandemic, team activities were impeded and became stagnant. According to the Ministry of Health, Labour and Welfare's report on "Number of medical institutions accepting COVID-19 patients by national, public, and private institutions and the percentage of acceptances" as of November 2020, the acceptance rates of COVID-19 patients at national and public medical institutions are high at 58% and 75%, respectively, while the rate at private medical institutions is low at 17% [23]. This study was not able to collect data on the acceptance of COVID-19 patients at participating facilities. However, as shown in Table 1, we speculated that the restriction on the admission of COVID-19 patients may have been one of the factors that reduced the incidence of CLABSI since the non-NST group had a higher proportion of private medical institutions than the NST group.
This study has several limitations. First, it was a retrospective observational study, and we could not adjust for all confounding factors affecting the incidence of CLABSI.
Second, although there are numerous NST facilities operating nationwide, we included a small number of facilities which limits generalizability. Third, the incidence of CLABSI may be affected by various factors, including the infection control system, and further study is needed to prove the effect is limited to NST. Multi-center studies accounting for these limitations are warranted to focus on the coordination system of medical teams such as the NST and infection control teams. Elucidating the effectiveness of the NST in preventing the occurrence of CLABSI could inform the improvement of the NST activity with respect to the fight against emerging and unknown infections.
In our study, the incidence of CLABSI during the COVID-19 pandemic was lower in the NST group than in the non-NST group, which indicates the effectiveness of the NST in preventing the occurrence of CLABSI.
Acknowledgements
We thank Tomomi Jinnouchi in Makino Rehabilitation Hospital; Naohito Nakamura in Tosei General Hospital; Mizuho Kabeya in Fuefuki Central Hospital; Rie Nishida and Takaki Kanie in, Fujita Health University hospital; Sayaka Taji in Minoh City Hospital; Fumie Yamada in Tsukuba Medical Center Hospital; Takeshi Yoshimi in Japanese Red Cross Medical Center; Toshihiro Nakanishi in Clinical Safety Management Group, Toyota Memorial Hospital, Toyota Motor Corporation; Masaya Ito in Hashima City Hospital; Mayu Shibata in Hamamatsu Red Cross Hospital; Hiroshi Ishihara in the Department of Pharmacy, Japanese Red Cross Gifu Hospital; Hiroko Kimbara in Public Central Hospital of Matto Ishikawa; Shin Inoue in Oita Oka Hospital; Keisuke Suzuki in Taito Hospital; Masashi Fujita in Komono Kosei Hospital; Namiki Makiko in Funabashi Municipal Medical Center; Yukari Takeno in Oota Hospital; Yoshihiro Uekuzu in the Department of Pharmacy, Fujita Health University Okazaki Medical Center; Yuka Aimono in Hitachi General Hospital; Maho Tatsumi in Kakogawa Central City Hospital; Takashi Matsuzaki in the Department of Pharmacy, St. Marianna University, Yokohama Seibu Hospital; Hiroshi Shinonaga in the Department of Pharmaceutical Services, Mitoyo General Hospital; Taku Ito in the Department of Pharmacy, Tenshi Hospital; Miki Kawasaki in Neuromuscular Center Yoshimizu Hospital; Yoichi Shibata in National Hospital Organization Yamagata Hospital; Hasegawa Mio in Fujiikai Kashibaseiki Hospital; Akiko Sasaki in Hibino Hospital; Hideki Yamaguchi in U Yakkyoku; Kyoko Saito in Kakio Memorial Hospital; Shigeki Ikushima in Nara Prefecture General Medical Center; Hitoshi Yagi in Kanto Rosai Hospital; Eiji Hotta in Fukui-ken Saiseikai Hospital; Yusuke Takata in Ehime University Hospital; Hiroki Asakawa in Hokuto Municipal Koyo Hospital; Yoji Ito in Saiki Central Hospital; Mariko Nakamichi in Haradoi Hospital; Takuya Sugahara in Yamagata City Hospital Saiseikan; Daisuke Maekawa in Ikoma City Hospital; Yoshikazu Watanabe in Saiseikai Utsunomiya Hospital; Tsukasa Sekimoto in Hasegawa Hospital; and Kyouko Mori in Fureai-Kamakura Hospital for providing data regarding patients with CRBSI. The authors thank Editage (https://www.editage.com/) for editing and reviewing the manuscript for the English language. This work was supported by the Japanese Society for Clinical Nutrition and Metabolism.
Abbreviations
- NST
Nutrition support team
- CLABSI
Central line-associated bloodstream infection
- COVID-19
Coronavirus disease
- ICP
Infection control prevention additional medical fee
Akihiko Futamura
Fujita Health University Nanakuri Memorial Hospital, Pharmacy Department Chief, Director of the Japanese Society for Parenteral and Enteral Nutrition Therapy, Chairman, Pharmacist Section.
Authors’ contributions
AF was the chief investigator. Contributor AF was responsible for organizing and coordinating the trial. AF, NM, MM, JI, HM, AS, KM, HO, YT, KH, and MU conceived and designed the study. AF and TK collected data. AF and TK performed the statistical analyses. AF drafted the manuscript. NM, MM, JI, HM, AS, KM, HO, YT, KH, and MU reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Japanese Society for Parenteral and Enteral Nutrition Therapy [grant number not available]. The funder had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Data availability
Data and materials are available through Fujita Health University Research Electronic Data Capture.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Fujita Health University (HM22-418). The requirement for informed consent was waived given the retrospective design of the study. We used an opt-out method based on the ethics committee approval requirements and national ethical guidelines; additionally, patients could obtain information regarding the study from the institution’s website.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This article has been updated to correct the acknowledgement section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/8/2025
A Correction to this paper has been published: 10.1186/s40780-024-00407-0
References
- 1.Stevens V, Geiger K, Concannon C, Nelson RE, Brown J, Dumyati G. Inpatient costs、 mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clin Microbiol Infect. 2014;20:O318–24. 10.1111/1469-0691.12407. [DOI] [PubMed] [Google Scholar]
- 2.Karagiannidou S, Zaoutis T, Maniadakis N, Papaevangelou V, Kourlaba G. Attributable length of stay and cost for pediatric and neonatal central line-associated bloodstream infections in Greece. J Infect Public Health. 2019;12:372–9. 10.1016/j.jiph.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552–7. 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- 4.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–601. 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 5.Meguro E, Yamane N, Yamamoto A, Kaji M. Present conditions including medical cost with central line-associated bloodstream infection. Jpn J Environ Infect. 2018;33:269–75. 10.4058/jsei.33.269. [Google Scholar]
- 6.Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery. 1968;64:134–42. [PubMed] [Google Scholar]
- 7.Colley R. Education of the hospital staff, total Pareteral nutrition, Fischer JE, editor. Boston: Little, Brown & Company, p. 111–25; 1976
- 8.Yamaguchi S, Okada A, Sunaga S, Ikeda Kurakawa K, Yamauchi T, Nangaku M, et al. Impact of COVID-19 pandemic on healthcare service use for non-COVID-19 patients in Japan: retrospective cohort study. BMJ Open. 2022;12:e060390. 10.1136/bmjopen-2021-060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuo T, Taki F, Kobayashi D, Jinta T, Suzuki C, Ayabe A, et al. Health care worker burnout after the first wave of the coronavirus disease 2019 (COVID-19) pandemic in Japan. J Occup Health. 2021;63:e12247. 10.1002/1348-9585.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullangi S, Aviki EM, Chen Y, Robson M, Hershman DL. Factors associated with cancer treatment delay among patients diagnosed with COVID-19. JAMA Netw Open. 2022;5:e2224296. 10.1001/jamanetworkopen.2022.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hospital Infection Control Guidelines 2018 Edition, March 2020 Supplementary Edition, Jiho, published December 25, 2020). https://kansen.med.nagoya-u.ac.jp/general/gl/gl2018-2/gl2018-2.html. Accessed 20 Sept 2024.
- 12.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792–801. 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 14.Song IA, Lee K, Lee S, Kim K, Oh TK. Implementation of a multidisciplinary nutritional support team and clinical outcomes in critically ill patients with COVID-19. Clin Nutr. 2024;43:315–21. 10.1016/j.clnu.2023.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Boni S, Sartini M, Del Puente F, Adriano G, Blasi Vacca EB, Bobbio N, et al. Innovative approaches to monitor central line associated bloodstream infections (CLABSIs) bundle efficacy in Intensive Care Unit (ICU): role of device standardized infection rate (dSIR) and standardized utilization ratio (SUR)—an Italian experience. J Clin Med. 2024;13. 10.3390/jcm13020396 [DOI] [PMC free article] [PubMed]
- 16.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terazawa E, Nagase K, Masue T, Niwa Y, Fukao I, Shimonaka H, Yokoi T, Kondoh N, Dohi S. Anaphylactic shock associated with a central venous catheter impregnated with chlorhexidine and silver sulfadiazine. Masui. 1998;47:556–61. [PubMed] [Google Scholar]
- 18.Hoshino Y, Watanabe T, Saijo F, Kanno K. Exploration of Risk factors for central-line-associated bloodstream infection. J Jpn Soc Hosp Pharm. 2021;57:548–52. [Google Scholar]
- 19.Melly MA, Meng HC, Schaffner W. Microbiol growth in lipid emulsions used in parenteral nutrition. Arch Surg. 1975;110:1479–81. 10.1001/archsurg.1975.01360180049010. [DOI] [PubMed] [Google Scholar]
- 20.Avila-Figueroa C, Goldmann DA, Richardson DK, Gray JE, Ferrari A, Freeman J. Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns. Pediatr Infect Dis J. 1998;17:10–7. 10.1097/00006454-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Granda MJ, Carrillo CS, Rabadán PM, Valerio M, Olmedo M, Muñoz P, et al. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: a plea for control. J Hosp Infect. 2022;119:149–54. 10.1016/j.jhin.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verberk JDM, van der Kooi TII, Kampstra NA, Reimes N, van Rooden SM, Hopmans TEM, et al. Healthcare-associated infections in Dutch hospitals during the COVID-19 pandemic. Antimicrob Resist Infect Control. 2023;12:2. 10.1186/s13756-022-01201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiscal year: COVID-19 and social security; 2021 [Ministry of Health, Labour and Welfare white paper]. https://www.mhlw.go.jp/stf/wp/hakusyo/kousei/20/backdata/2-3-1-12.html. Accessed 20 Sept 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available through Fujita Health University Research Electronic Data Capture.