Abstract
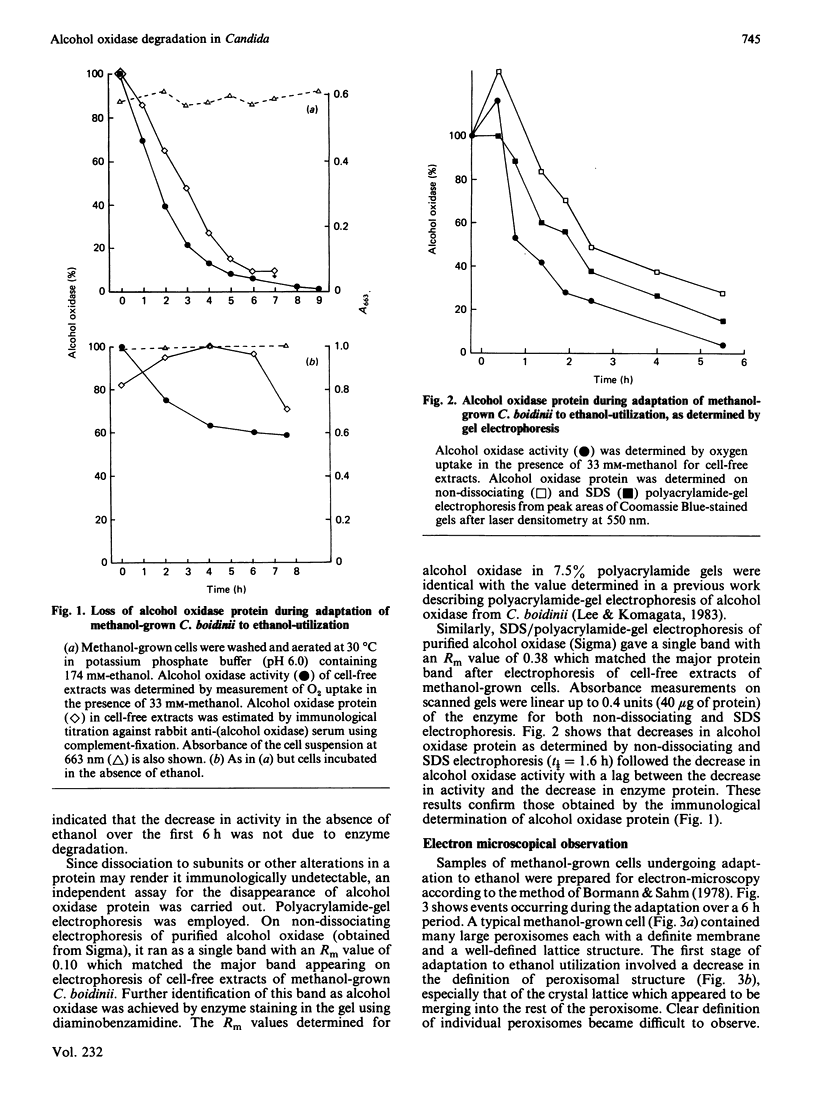
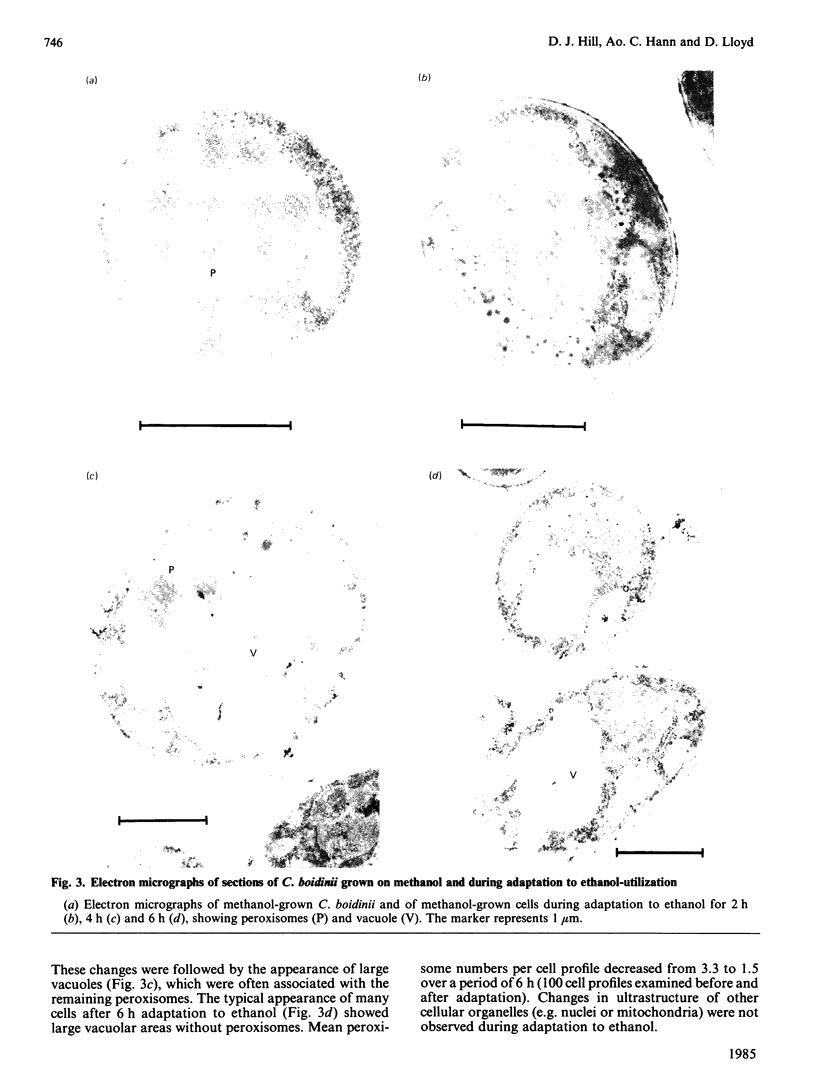
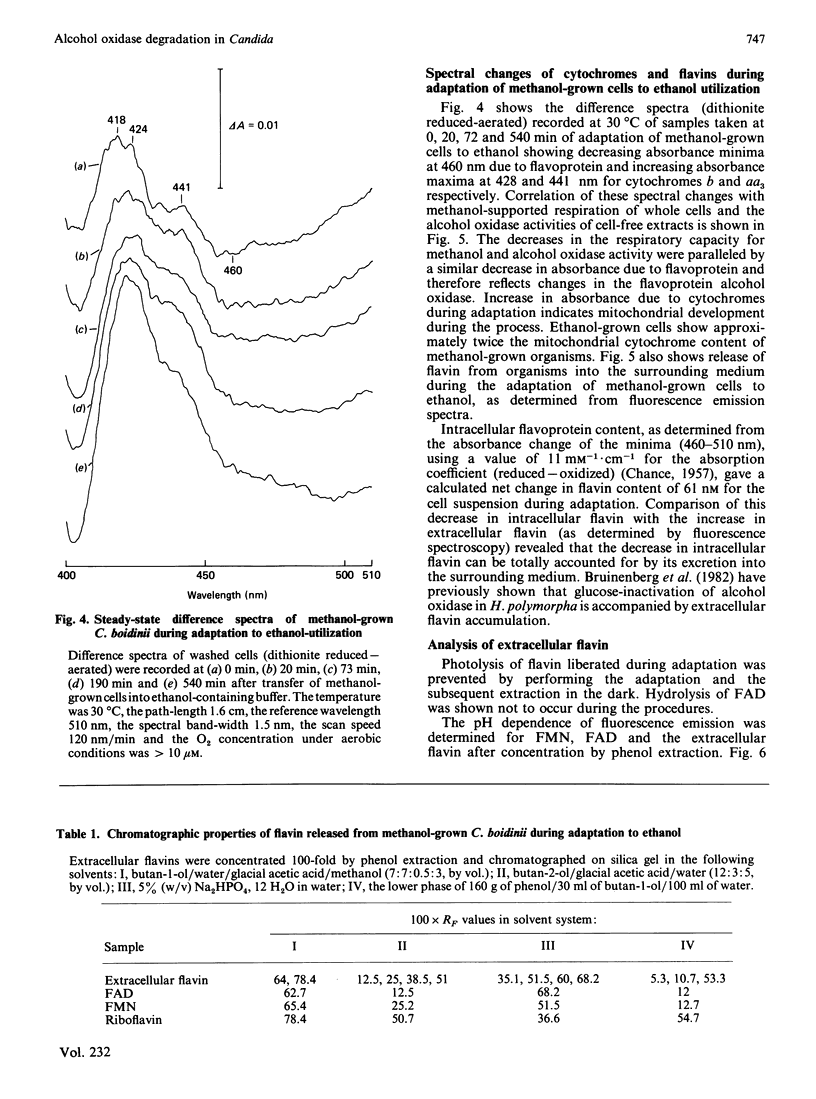
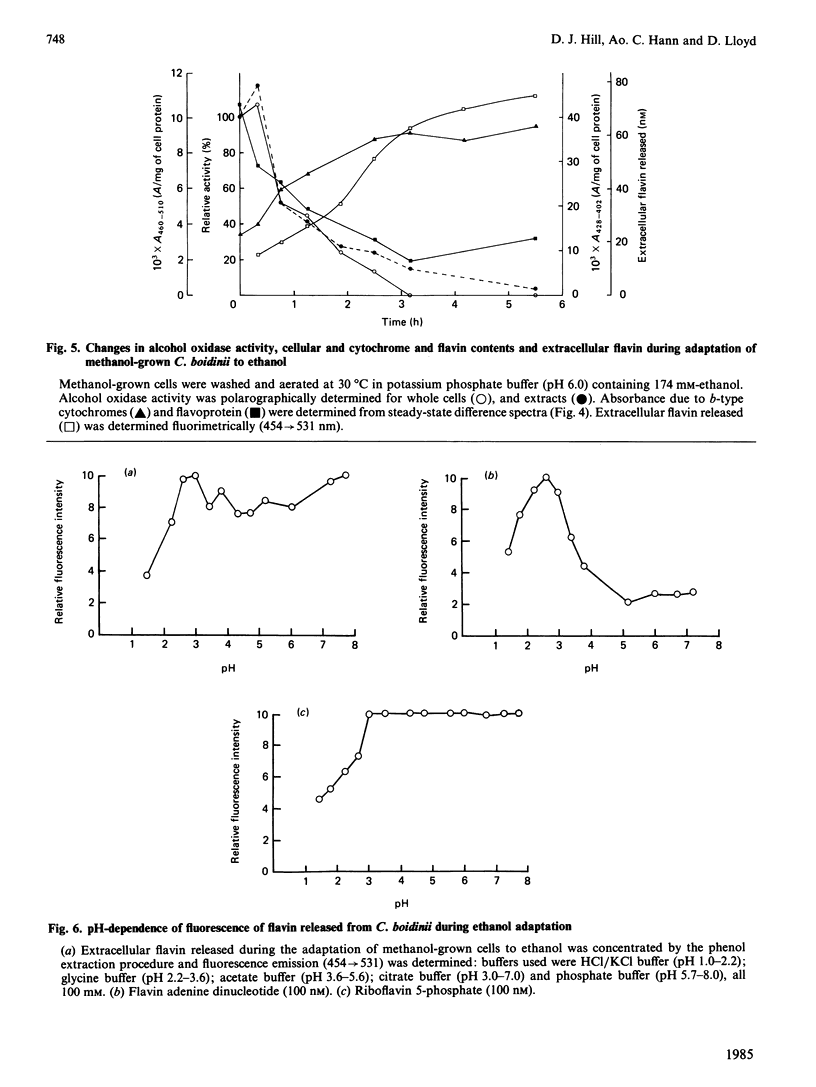
Adaptation of methanol-grown C. boidinii to ethanol-utilization in non-growing cells resulted in decreased activity of the peroxisomal enzyme alcohol oxidase. Re-appearance of alcohol oxidase activity was dependent on protein synthesis de novo. Degradation of alcohol oxidase protein was shown to parallel the decrease in activity. Adaptation of methanol-grown cells to ethanol-utilization resulted in increased absorbance due to cytochromes and decreased absorbance due to flavoprotein. Decrease in alcohol oxidase activity was associated with loss of the flavin coenzyme, FAD, from the organisms and the appearance of flavins (FAD, FMN, riboflavin) in the surrounding medium. Electron microscopic observations showed that general degradation of whole peroxisomes rather than specific loss of crystalline cores (alcohol oxidase protein) occurred during the adaptation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormann C., Sahm H. Degradation of microbodies in relation to activities of alcohol oxidase and catalase in Candida boidinii. Arch Microbiol. 1978 Apr 27;117(1):67–72. doi: 10.1007/BF00689353. [DOI] [PubMed] [Google Scholar]
- Degn H., Lundsgaard J. S., Petersen L. C., Ormicki A. Polarographic measurement of steady state kinetics of oxygen uptake by biochemical samples. Methods Biochem Anal. 1980;26:47–77. doi: 10.1002/9780470110461.ch2. [DOI] [PubMed] [Google Scholar]
- Haywood G. W., Large P. J. Microbial oxidation of amines. Distribution, purification and properties of two primary-amine oxidases from the yeast Candida boidinii grown on amines as sole nitrogen source. Biochem J. 1981 Oct 1;199(1):187–201. doi: 10.1042/bj1990187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A. The mechanism, role and control of the inactivation of glutamate dehydrogenases in yeast. Biochem Soc Trans. 1982 Oct;10(5):328–329. doi: 10.1042/bst0100328. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazón M. J., Gancedo J. M., Gancedo C. Inactivation of yeast fructose-1,6-bisphosphatase. In vivo phosphorylation of the enzyme. J Biol Chem. 1982 Feb 10;257(3):1128–1130. [PubMed] [Google Scholar]
- Roggenkamp R., Sahm H., Hinkelmann W., Wagner F. Alcohol oxidase and catalase in peroxisomes of methanol-grown Candida boidinii. Eur J Biochem. 1975 Nov 1;59(1):231–236. doi: 10.1111/j.1432-1033.1975.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Douma A., Harder W., Osumi M. Degradation and turnover of peroxisomes in the yeast Hansenula polymorpha induced by selective inactivation of peroxisomal enzymes. Arch Microbiol. 1983 Jun;134(3):193–203. doi: 10.1007/BF00407757. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Van Dijken J. P., Harder W. The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts. Adv Microb Physiol. 1983;24:1–82. doi: 10.1016/s0065-2911(08)60384-7. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- Wolf D. H. Control of metabolism in yeast and other lower eukaryotes through action of proteinases. Adv Microb Physiol. 1980;21:267–338. doi: 10.1016/s0065-2911(08)60358-6. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]