Abstract
Glycophorin A was purified from the erythrocyte membranes of blood group Cad, Sd(a+) and Sd(a-) donors and the oligosaccharide alditols, obtained after alkaline borohydride degradation, separated by h.p.l.c. on an alkylamine silica gel column, were characterized by sugar analysis. Structure determination of the major acid components by methylation analysis, g.l.c.-m.s. and 1H-n.m.r. indicated that the three blood group Cad red cells under study (samples Cad., Bui. and Des.) carry the same pentasaccharide GalNAc(beta 1-4)[NeuAc(alpha 2-3)]Gal(beta 1-3)[NeuAc(alpha 2-6)]GalNAc -ol(Cad determinant) but in different amounts. This pentasaccharide, however, was absent from glycophorin A of Sd(a+) and Sd (a-) donors, suggesting that the Sda determinant is not associated with glycophorins. It was calculated that glycophorin A from the original Cad donor (Cad.) carries about 12 O-glycosidically linked pentasaccharide chains per molecule whereas only 2-3 of these chains were present in the samples from the two other unrelated Cad individuals (Bui. and Des.) It is well known from quantitative agglutination studies that the proportion of red cells which can be agglutinated by the Dolichos biflorus lectin varies from one Cad blood sample to another. Some are completely agglutinated (Cad. donor) whereas others are only partially agglutinated (Bui. and Des. donors) suggesting that some red cells might not carry the Cad determinants. From the results presented above and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis studies it is suggested that Cad red cells from Bui. and Des. do not carry a mixture of glycophorin A molecules with or without the Cad pentasaccharides but a spectrum of glycoprotein molecules with varying amounts of Cad determinants.
Full text
PDF
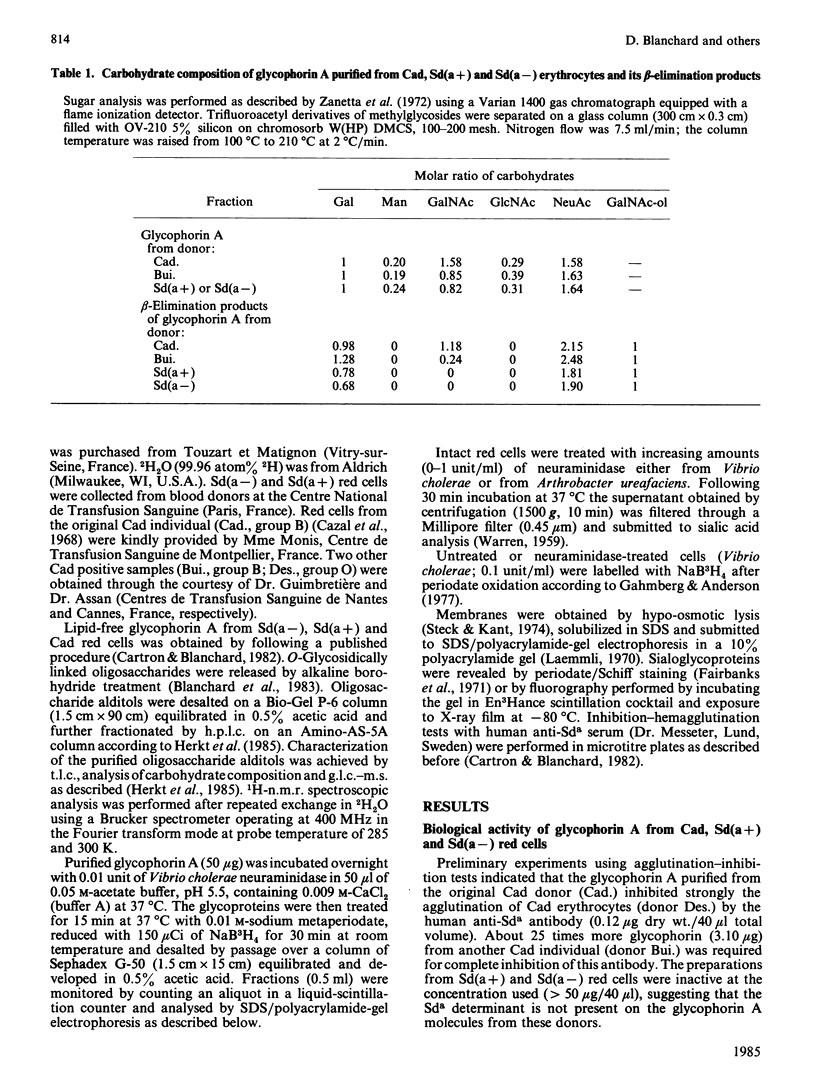
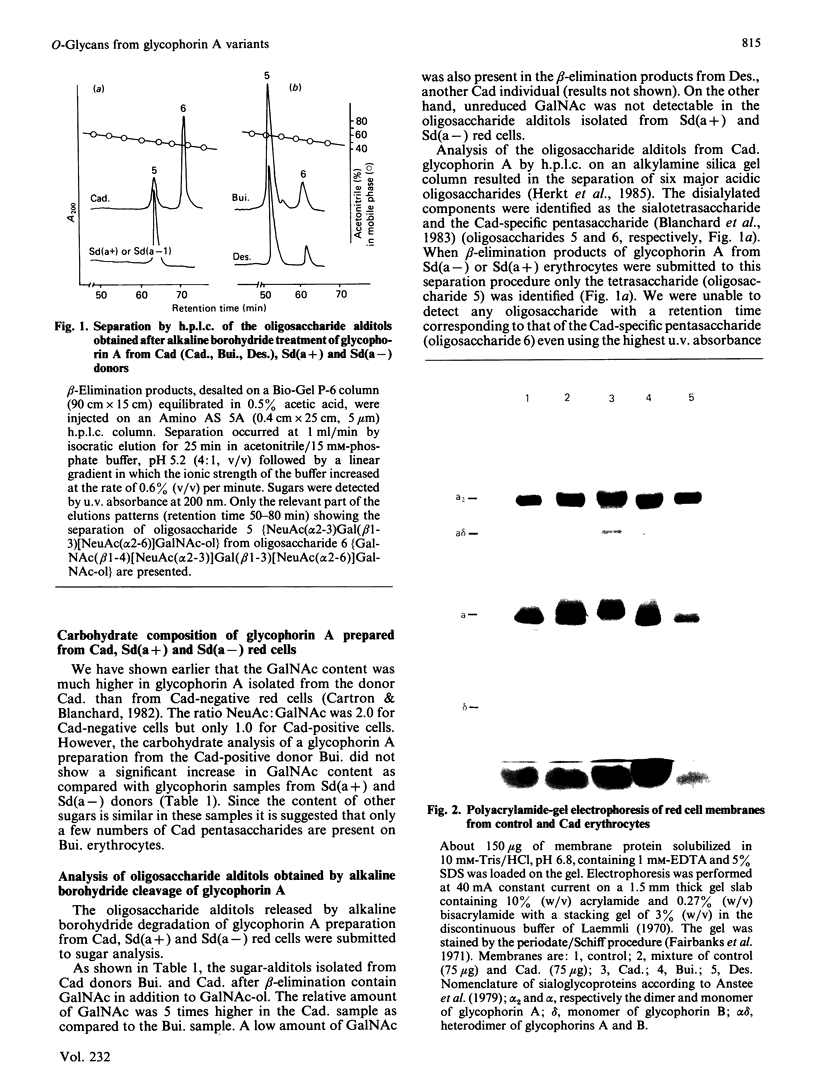


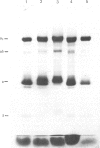
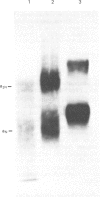
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Mawby W. J., Tanner M. J. Abnormal blood-group-Ss-active sialoglycoproteins in the membrane of Miltenberger class III, IV and V human erythrocytes. Biochem J. 1979 Nov 1;183(2):193–203. doi: 10.1042/bj1830193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D., Cartron J. P., Fournet B., Montreuil J., van Halbeek H., Vliegenthart J. F. Primary structure of the oligosaccharide determinant of blood group Cad specificity. J Biol Chem. 1983 Jun 25;258(12):7691–7695. [PubMed] [Google Scholar]
- Blanchard D., Piller F., Gillard B., Marcus D., Cartron J. P. Identification of a novel ganglioside on erythrocytes with blood group Cad specificity. J Biol Chem. 1985 Jul 5;260(13):7813–7816. [PubMed] [Google Scholar]
- Cartron J. P., Blanchard D. Association of human erythrocyte membrane glycoproteins with blood-group Cad specificity. Biochem J. 1982 Dec 1;207(3):497–504. doi: 10.1042/bj2070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron J. P., Nurden A. T., Blanchard D., Lee H., Dupuis D., Salmon C. The Tn receptors of human red cells and platelets. Rev Fr Transfus Immunohematol. 1980 Nov;23(5):613–628. doi: 10.1016/s0338-4535(80)80164-4. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Prou O., Luilier M., Soulier J. P. Susceptibility to invasion by Plasmodium falciparum of some human erythrocytes carrying rare blood group antigens. Br J Haematol. 1983 Dec;55(4):639–647. doi: 10.1111/j.1365-2141.1983.tb02846.x. [DOI] [PubMed] [Google Scholar]
- Cazal P., Monis M., Bizot M. Les antigènes Cad et leurs rapports avec les antigènes. Rev Fr Transfus. 1971 Sep;14(3):321–334. doi: 10.1016/s0035-2977(71)80023-8. [DOI] [PubMed] [Google Scholar]
- Cazal P., Monis M., Caubel J., Brives J. Polyagglutinabilité héréditaire dominante: antigène privé (Cad) correspondant à un anticorps public et à une lectine de Dolichos biflorus. Rev Fr Transfus. 1968 Oct 3;11(3):209–221. doi: 10.1016/s0035-2977(68)80050-1. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Kornfeld S. A murine cytotoxic T lymphocyte cell line resistant to Vicia villosa lectin is deficient in UDP-GalNAc:beta-galactose beta 1,4-N-acetylgalactosaminyltransferase. J Biol Chem. 1984 Oct 25;259(20):12536–12542. [PubMed] [Google Scholar]
- Donald A. S., Yates A. D., Soh C. P., Morgan W. T., Watkins W. M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem Biophys Res Commun. 1983 Sep 15;115(2):625–631. doi: 10.1016/s0006-291x(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Herkt F., Parente J. P., Leroy Y., Fournet B., Blanchard D., Cartron J. P., van Halbeek H., Vliegenthart J. F. Structure determination of oligosaccharides isolated from Cad erythrocyte membranes by permethylation analysis and 500-MHz 1H-NMR spectroscopy. Eur J Biochem. 1985 Jan 2;146(1):125–129. doi: 10.1111/j.1432-1033.1985.tb08628.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez M., Gerbal A., Bony V., Salmon C. Cad antigen: comparative study of 50 samples. Vox Sang. 1975;28(4):305–313. doi: 10.1111/j.1423-0410.1975.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Sanger R., Gavin J., Tippett P., Teesdale P., Eldon K. Plant agglutinin for another human blood-group. Lancet. 1971 May 29;1(7709):1130–1130. doi: 10.1016/s0140-6736(71)91865-4. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F. Guinea-pig kidney beta-N-acetylgalactosaminyltransferase towards Tamm-Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem J. 1983 Dec 1;215(3):483–489. doi: 10.1042/bj2150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]