Abstract
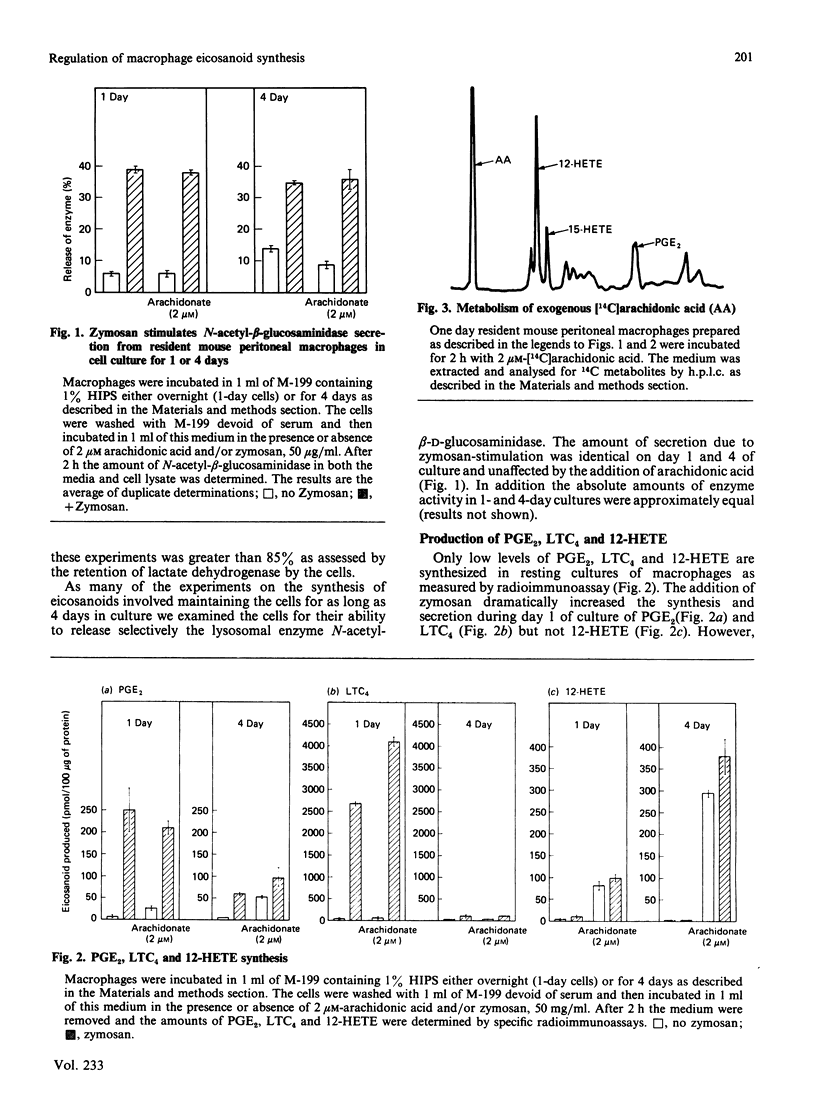
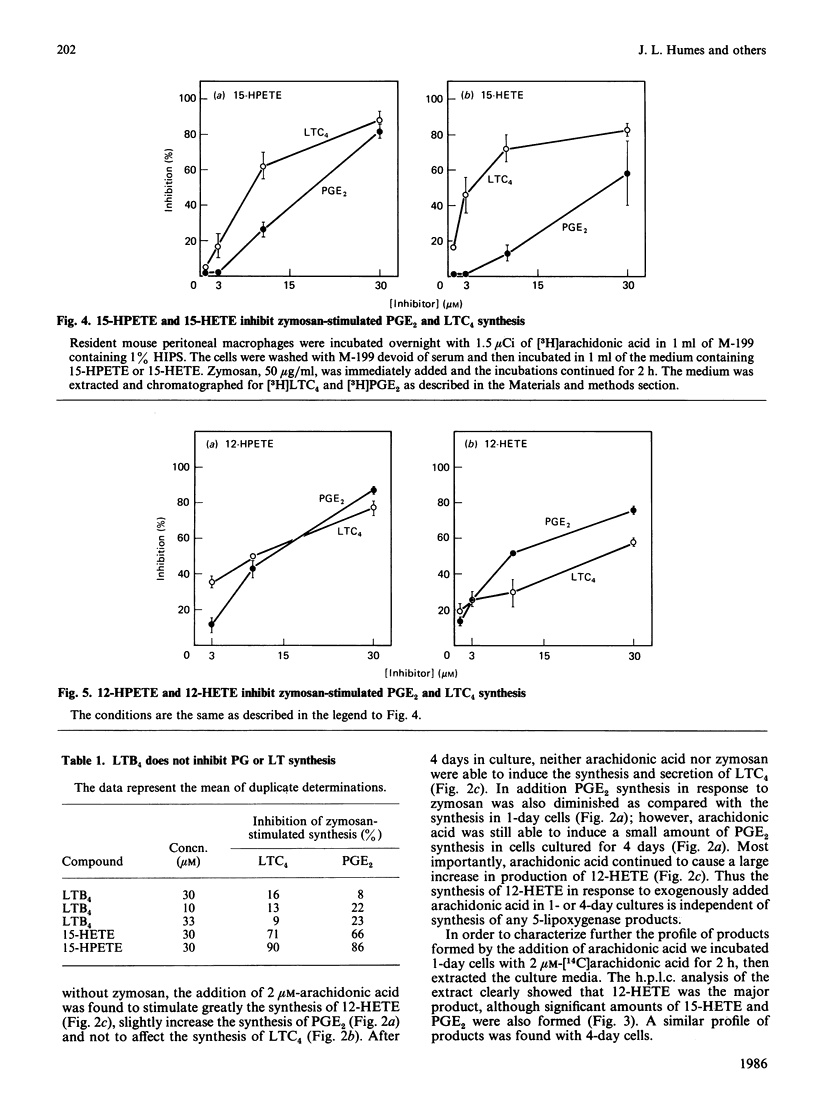
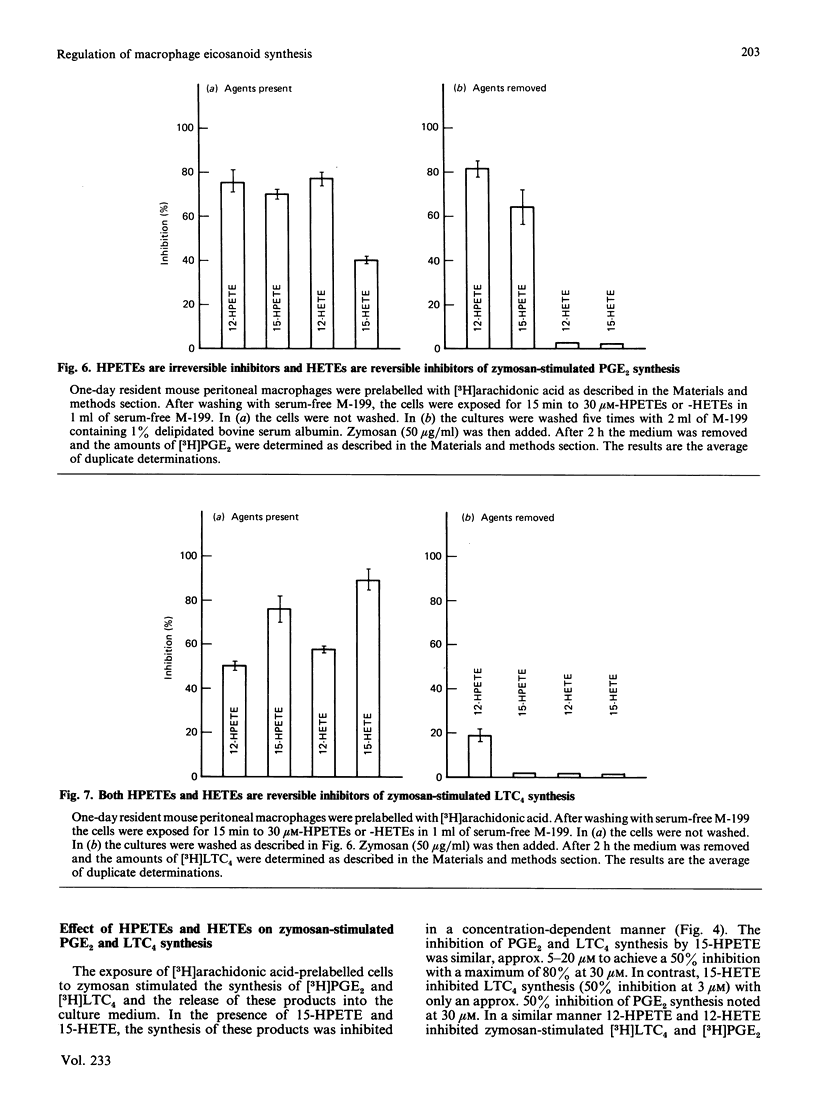
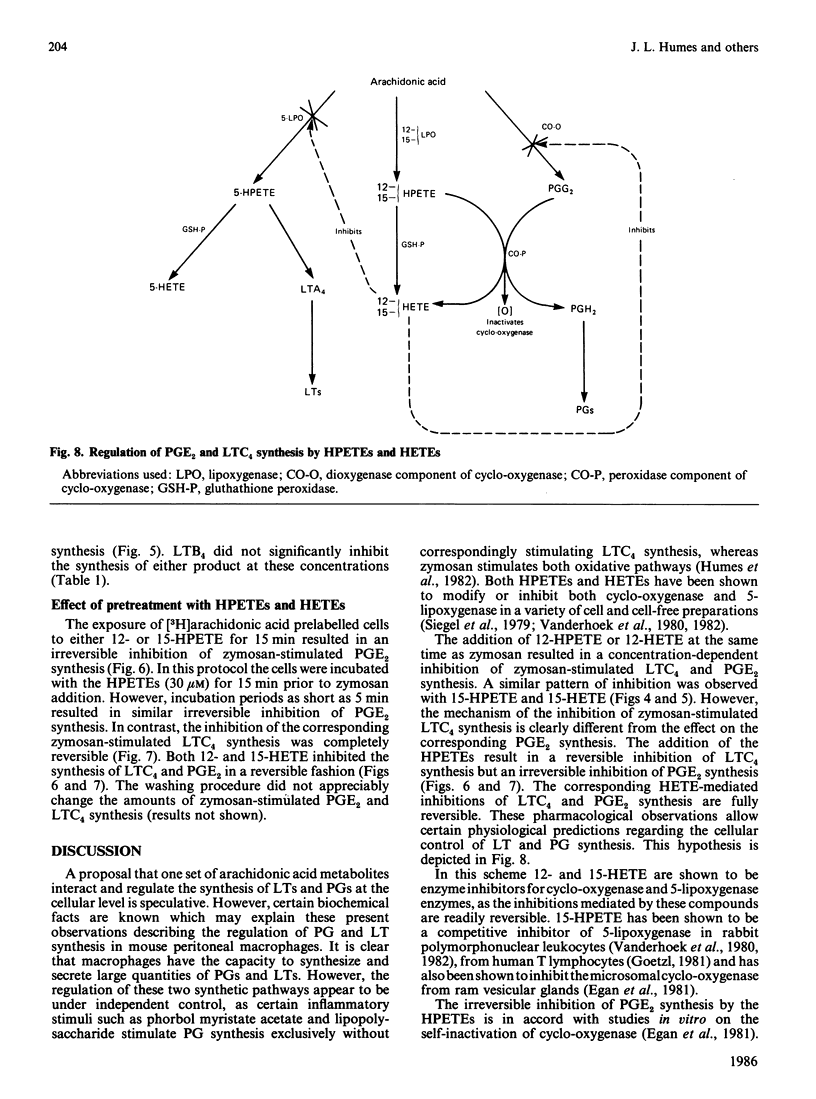
Resident mouse peritoneal macrophages when exposed to zymosan during the first day of cell culture synthesize and secrete large amounts of prostaglandin E2 (PGE2) and leukotriene C4 (LTC4), the respective products of cyclo-oxygenase- and 5-lipoxygenase-catalysed oxygenations of arachidonic acid. Under these conditions of cell stimulation only small amounts of hydroxyeicosatetraenoic acids (HETEs) are concomitantly produced. However, exogenously added arachidonic acid is metabolized to large amounts of 12- and 15-HETE and only relatively small amounts of PGE2. No LTC4 is formed under these conditions. In contrast, resident mouse peritoneal macrophages in cell culture for 4 days synthesized less PGE2 and LTC4 when exposed to zymosan. However, these macrophage populations continue to synthesize 12-HETE from exogenously added arachidonic acid. Zymosan induced the secretion of a lysosomal enzyme, N-acetyl-beta-glucosaminidase, equally in both 1- and 4-day cultures. Both 12- and 15-hydroperoxyeicosatetraenoic acids (HPETEs), the precursors of 12- and 15-HETE, were found to be irreversible inhibitors of the cyclo-oxygenase pathway and reversible inhibitors of the 5-lipoxygenase pathway in macrophages. 15-HETE were found to be reversible inhibitors of both pathways. Thus the oxidation of arachidonic oxidation of arachidonic acid to both prostaglandins and leukotrienes may be under intracellular regulation by products of 12- and 15-lipoxygenases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Bryant R. W., Low C. E., Pupillo M. B., Vanderhoek J. Y. Regulation of T-lymphocyte mitogenesis by the leukocyte product 15-hydroxy-eicosatetraenoic acid (15-HETE). Cell Immunol. 1982 Feb;67(1):112–120. doi: 10.1016/0008-8749(82)90203-9. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Lamb B., Marinari L., Kreft A. F., Lewis A. J. Modulation by hydroxyeicosatetraenoic acids (HETEs) of arachidonic acid metabolism in mouse resident peritoneal macrophages. Eur J Pharmacol. 1985 Jan 2;107(2):215–222. doi: 10.1016/0014-2999(85)90061-5. [DOI] [PubMed] [Google Scholar]
- Crawford C. G., van Alphen G. W., Cook H. W., Lands W. E. The effect of precursors, products, and product analogs of prostaglandin cyclooxygenase upon iris sphincter muscle. Life Sci. 1978 Sep 25;23(12):1255–1262. doi: 10.1016/0024-3205(78)90503-9. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Baptista E. M., Kennicott K. L., VandenHeuvel W. J., Walker R. W., Fagerness P. E., Kuehl F. A., Jr Oxidation reactions by prostaglandin cyclooxygenase-hydroperoxidase. J Biol Chem. 1981 Jul 25;256(14):7352–7361. [PubMed] [Google Scholar]
- Goetzl E. J. Selective feed-back inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochem Biophys Res Commun. 1981 Jul 30;101(2):344–350. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- Gualde N., Chable-Rabinovitch H., Motta C., Durand J., Beneytout J. L., Rigaud M. Hydroperoxyeicosatetraenoic acids. Potent inhibitors of lymphocyte responses. Biochim Biophys Acta. 1983 Mar 1;750(3):429–433. doi: 10.1016/0005-2760(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Ham E. A., Egan R. W., Soderman D. D., Gale P. H., Kuehl F. A., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979 Apr 10;254(7):2191–2194. [PubMed] [Google Scholar]
- Hammarström S., Hamberg M., Duell E. A., Stawiski M. A., Anderson T. F., Voorhees J. J. Glucocorticoid in inflammatory proliferative skin disease reduces arachidonic and hydroxyeicosatetraenoic acids. Science. 1977 Sep 2;197(4307):994–996. doi: 10.1126/science.887938. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hamberg M., Samuelsson B., Duell E. A., Stawiski M., Voorhees J. J. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. C., Lombardo D. L., Girard Y., Maycock A. L., Rokach J., Rosenthal A. S., Young R. N., Egan R. W., Zweerink H. J. Measuring leukotrienes of slow reacting substance of anaphylaxis: development of a specific radioimmunoassay. J Immunol. 1983 Jul;131(1):429–433. [PubMed] [Google Scholar]
- Hemler M. E., Graff G., Lands W. E. Accelerative autoactivation of prostaglandin biosynthesis by PGG2. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1325–1331. doi: 10.1016/0006-291x(78)91148-8. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Burger S., Galavage M., Kuehl F. A., Jr, Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The diminished production of arachidonic acid oxygenation products by elicited mouse peritoneal macrophages: possible mechanisms. J Immunol. 1980 May;124(5):2110–2116. [PubMed] [Google Scholar]
- Humes J. L., Sadowski S., Galavage M., Goldenberg M., Subers E., Bonney R. J., Kuehl F. A., Jr Evidence for two sources of arachidonic acid for oxidative metabolism by mouse peritoneal macrophages. J Biol Chem. 1982 Feb 25;257(4):1591–1594. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maas R. L., Turk J., Oates J. A., Brash A. R. Formation of a novel dihydroxy acid from arachidonic acid by lipoxygenase-catalyzed double oxygenation in rat mononuclear cells and human leukocytes. J Biol Chem. 1982 Jun 25;257(12):7056–7067. [PubMed] [Google Scholar]
- Pace-Asciak C. R., Granström E., Samuelsson B. Arachidonic acid epoxides. Isolation and structure of two hydroxy epoxide intermediates in the formation of 8,11,12- and 10,11,12-trihydroxyeicosatrienoic acids. J Biol Chem. 1983 Jun 10;258(11):6835–6840. [PubMed] [Google Scholar]
- Rabinovitch H., Durand J., Rigaud M., Mendy F., Breton J. C. Transformation of arachidonic acid into monohydroxy-eicosatetraenoic acids by mouse peritoneal macrophages. Lipids. 1981 Jul;16(7):518–524. doi: 10.1007/BF02535050. [DOI] [PubMed] [Google Scholar]
- Rigaud M., Durand J., Breton J. C. Transfomration of arachidonic acid into 12-hydroxy-5,8,10,14-eicosatetraenoic acid by mouse peritoneal macrophages. Biochim Biophys Acta. 1979 May 25;573(2):408–412. doi: 10.1016/0005-2760(79)90074-2. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Cohn Z. A., Blackburn P., Manning J. M. Mouse peritoneal macrophages release leukotriene C in response to a phagocytic stimulus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4928–4932. doi: 10.1073/pnas.77.8.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Andreach M., Cohn Z. A. Resting macrophages produce distinct metabolites from exogenous arachidonic acid. J Exp Med. 1982 Feb 1;155(2):535–547. doi: 10.1084/jem.155.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Abrahams S. L., Porter N. A., Cuatrecasas P. Regulation of arachidonate metabolism via lipoxygenase and cyclo-oxygenase by 12-HPETE, the product of human platelet lipoxygenase. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1273–1280. doi: 10.1016/0006-291x(79)92146-6. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Bryant R. W., Bailey J. M. Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J Biol Chem. 1980 Nov 10;255(21):10064–10066. [PubMed] [Google Scholar]
- Vanderhoek J. Y., Bryant R. W., Bailey J. M. Regulation of leukocyte and platelet lipoxygenases by hydroxyeicosanoids. Biochem Pharmacol. 1982 Nov 1;31(21):3463–3467. doi: 10.1016/0006-2952(82)90627-x. [DOI] [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]


