Abstract
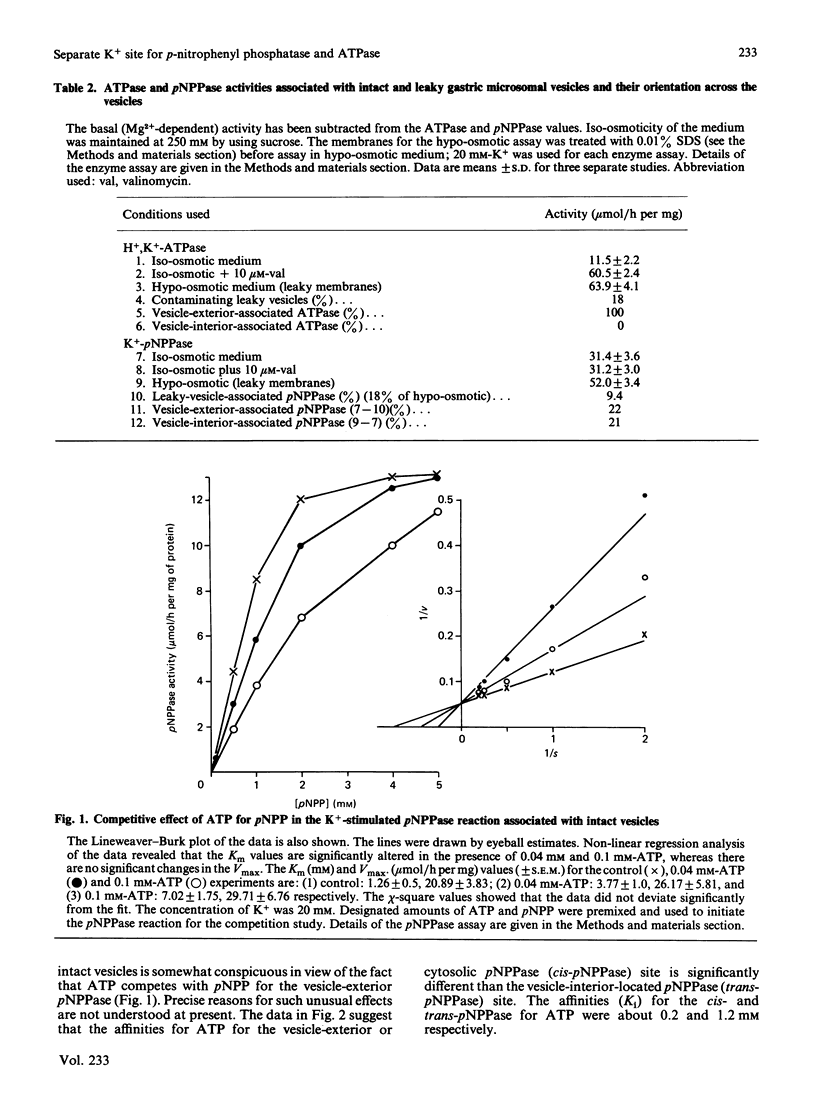
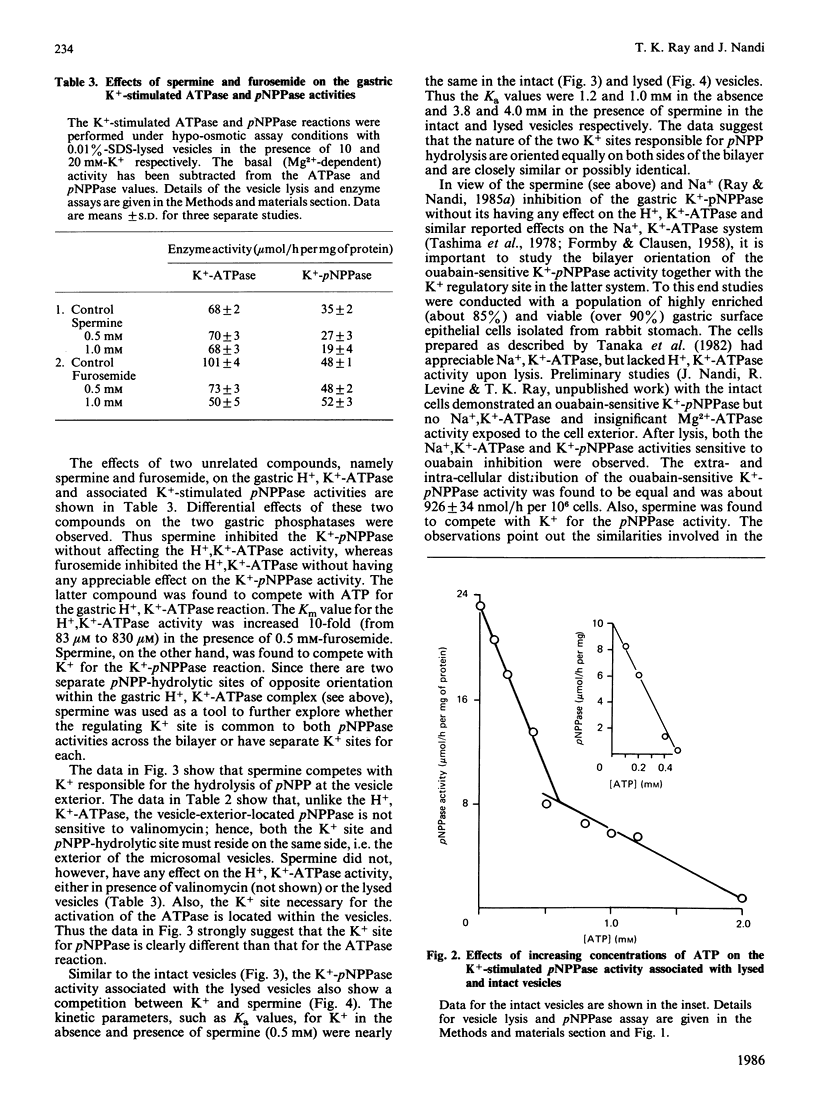
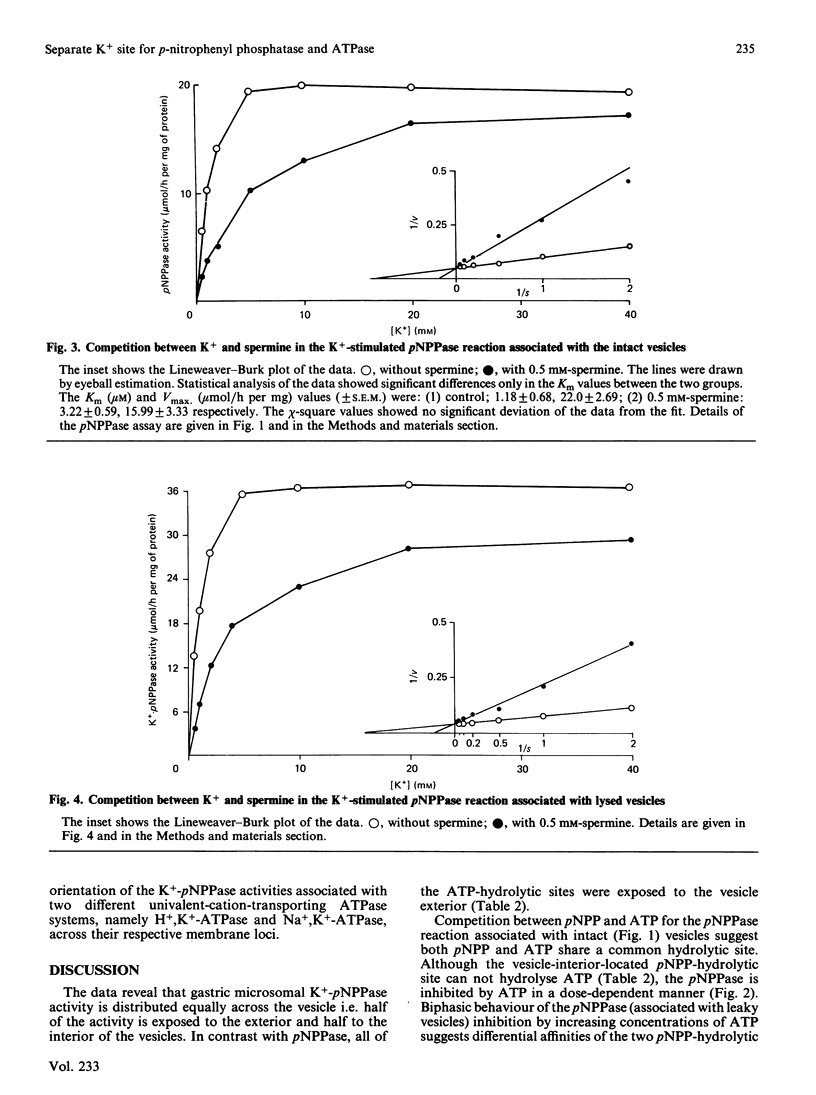
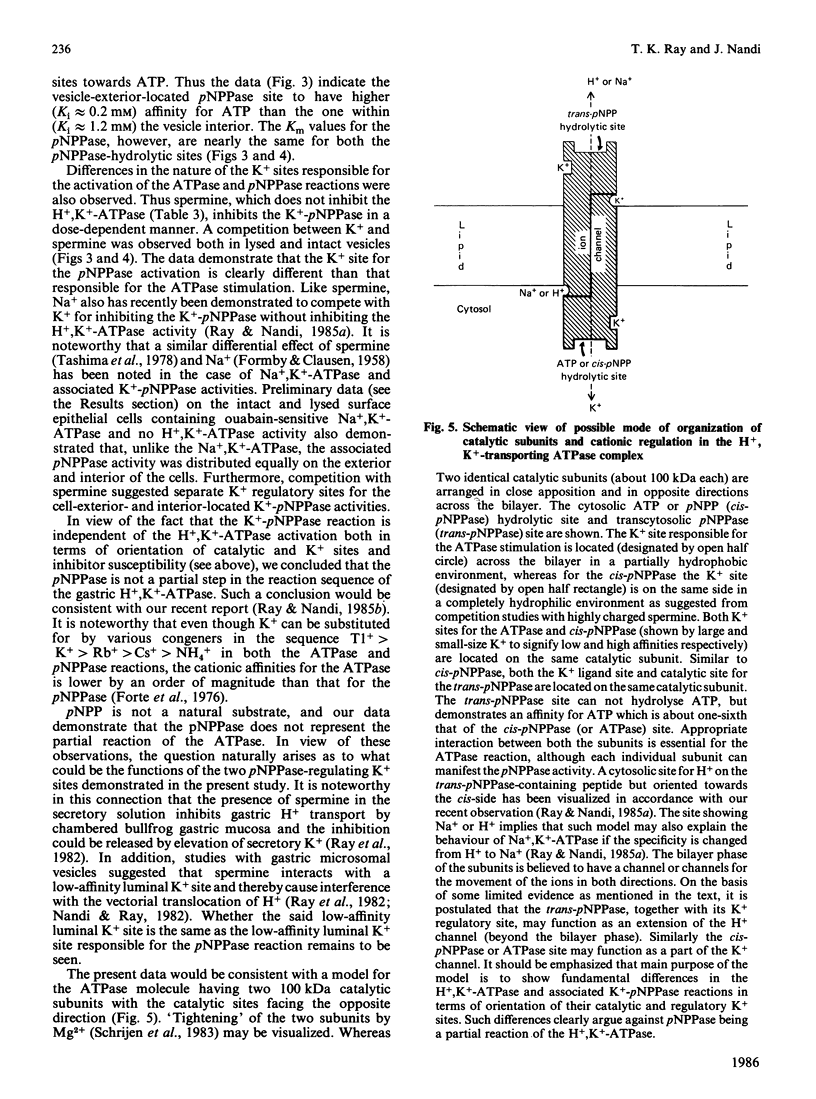
Studies with intact and lysed gastric microsomal vesicles demonstrate that there are two pNPP (p-nitrophenyl phosphate)-and one ATP-hydrolytic sites within the gastric H+, K+-ATPase [(H+ + K+)-transporting ATPase] complex. Whereas the ATPase site is located exclusively on the vesicle exterior, the pNPPase sites are distributed equally on both sides of the bilayer. Competition by ATP for the pNPPase reaction on the vesicle exterior suggests that both ATP and pNPP are hydrolysed at the same catalytic site present at the outside surface of the intact vesicles. However, a biphasic inhibition of the K+-pNPPase (K+-stimulated pNPPase) by ATP in the lysed vesicles suggest the pNPPase site of the vesicle interior to have very low affinity (Ki approximately equal to 1.2 mM) for ATP compared with the vesicle exterior (Ki approximately equal to 0.2 mM). Studies with spermine, which competes with K+ for the K+-pNPPase reaction without inhibiting the H+, K+-ATPase, suggest there are two separate K+ sites for the pNPPase reaction and another distinct K+ site for the ATPase reaction. In contrast with the K+ site for the ATPase, which is located opposite to the catalytic site across the bilayer, both the K+ and the catalytic site for the pNPPase are located on the same side. The data clearly demonstrate that the pNPPase is not a manifestation of the phosphatase step of the total H+, K+-ATPase reaction. The K+-pNPPase associated with the Na+, K+-ATPase also has properties strikingly similar to the gastric K+-pNPPase system, suggesting a resemblance in the basic operating principle of the two ion-transporting enzymes. A unified model has been proposed to explain the present data and many other observations reported in the literature for the ATPase-mediated transport of univalent cations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askari A. The effects of antibodies to Na+, K+-ATPase on the reactions catalyzed by the enzyme. Ann N Y Acad Sci. 1974;242(0):372–388. doi: 10.1111/j.1749-6632.1974.tb19104.x. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr Immunochemical characterization of a functional site of (Na+,K+)-ATPase. Biochemistry. 1984 May 8;23(10):2275–2281. doi: 10.1021/bi00305a029. [DOI] [PubMed] [Google Scholar]
- Formby B., Clausen J. Comparative studies on K -p-nitrophenylphosphatase, K -acylphosphatase and (Na + K)adenosinetriphosphatase in synaptosomes of rat brain. Hoppe Seylers Z Physiol Chem. 1968 Jul;349(7):909–919. doi: 10.1515/bchm2.1968.349.2.909. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Machen T. E., Obrink K. J. Mechanisms of gastric H+ and Cl- transport. Annu Rev Physiol. 1980;42:111–126. doi: 10.1146/annurev.ph.42.030180.000551. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Poulter J. L., Dykstra R., Rivas J., Lee H. C. Specific modification of gastric K+-stimulated ATPase activity by thimerosal. Biochim Biophys Acta. 1981 Jun 22;644(2):257–265. doi: 10.1016/0005-2736(81)90383-7. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Mendlein J., Sachs G. Interaction of fluorescein isothiocyanate with the (H+ + K+)-ATPase. Biochim Biophys Acta. 1983 May 26;731(1):9–15. doi: 10.1016/0005-2736(83)90391-7. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Nandi J., Meng-Ai Z., Ray T. K. Role of membrane-associated thiol groups in the functional regulation of gastric microsomal (H+ + K+)-transporting ATPase system. Biochem J. 1983 Sep 1;213(3):587–594. doi: 10.1042/bj2130587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi J., Ray T. K. Mechanism of action of gastric secretory inhibitors: effects of SCN- OCN-, NO-2, and NH+4 on (H+ + K+)-ATPase-mediated transport of H+ inside gastric microsomal vesicles. Arch Biochem Biophys. 1982 Jun;216(1):259–271. doi: 10.1016/0003-9861(82)90211-9. [DOI] [PubMed] [Google Scholar]
- Nandi J., Wright M. V., Ray T. K. Effects of phospholipase A2 on gastric microsomal H+, K+-ATPase system: role of "boundary lipids" and the endogenous activator protein. Biochemistry. 1983 Dec 6;22(25):5814–5821. doi: 10.1021/bi00294a020. [DOI] [PubMed] [Google Scholar]
- Nandi J., Wright M. V., Ray T. K. Mechanism of gastric antisecretory effects of nolinium bromide. Gastroenterology. 1983 Oct;85(4):938–945. [PubMed] [Google Scholar]
- Ottolenghi P. The relipidation of delipidated Na,K-ATPase. An analysis of complex formation with dioleoylphosphatidylcholine and with dioleoylphosphatidylethanolamine. Eur J Biochem. 1979 Aug 15;99(1):113–131. doi: 10.1111/j.1432-1033.1979.tb13238.x. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Fromm D. Cellular and subcellular aspects of the mechanism of gastric acid secretion. J Surg Res. 1981 Dec;31(6):496–505. doi: 10.1016/0022-4804(81)90188-8. [DOI] [PubMed] [Google Scholar]
- Ray T. K. Gastric K+-stimulated adenosine triphosphatase. Demonstration of an endogenous activator. FEBS Lett. 1978 Aug 1;92(1):49–52. doi: 10.1016/0014-5793(78)80719-4. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Nandi J., Dannemann A., Gordon G. B. Role of cholesterol in the structure and function of gastric microsomal vesicles. J Cell Biochem. 1983;21(2):141–150. doi: 10.1002/jcb.240210205. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Nandi J. Modulation of gastric H+,K+-transporting ATPase function by sodium. FEBS Lett. 1985 Jun 3;185(1):24–28. doi: 10.1016/0014-5793(85)80733-x. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Nandi J., Pidhorodeckyj N., Meng-Ai Z. Polyamines are inhibitors of gastric acid secretion. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1448–1452. doi: 10.1073/pnas.79.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. E., Ellory J. C., Glynn I. M. Radiation inactivation of (Na+ + K+)-ATPase. A small target size for the K+-occluding mechanism. Biochim Biophys Acta. 1981 Nov 6;648(2):284–286. doi: 10.1016/0005-2736(81)90045-6. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Saccomani G., Barcellona M. L., Sachs G. Reactivity of gastric (H+ + K+)-ATPase to N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. J Biol Chem. 1981 Dec 10;256(23):12405–12410. [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Schrijen J. J., Van Groningen-Luyben W. A., Nauta H., De Pont J. J., Bonting S. L. Studies on (K+ + H+)-ATPase. VI. Determination on the molecular size by radiation inactivation analysis. Biochim Biophys Acta. 1983 Jun 10;731(2):329–337. doi: 10.1016/0005-2736(83)90025-1. [DOI] [PubMed] [Google Scholar]
- Sen P. C., Ray T. K. Characterization of gastric-mucosal membranes. Distribution of lipid- and protein-associated amino groups across pig gastric microsomes. Biochem J. 1981 May 1;195(2):515–518. doi: 10.1042/bj1950515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Fromm D., Hill R. B., Kolis M. Isolation and viability of gastric mucosal surface cells of the rabbit. J Surg Res. 1982 Oct;33(4):265–279. doi: 10.1016/0022-4804(82)90039-7. [DOI] [PubMed] [Google Scholar]
- Tashima Y., Hasegawa M., Mizunuma H. Activation of Na+-K+-adenosine triphosphatase by spermine. Biochem Biophys Res Commun. 1978 May 15;82(1):13–18. doi: 10.1016/0006-291x(78)90569-7. [DOI] [PubMed] [Google Scholar]
- Tashima Y., Hasegawa M., Mizunuma H., Sakagishi Y. Specific effects of spermine on ouabain-sensitive and potassium-dependent phosphatase activity of kidney plasma membranes. Specificity of the potassium sites. Biochim Biophys Acta. 1977 May 12;482(1):1–10. doi: 10.1016/0005-2744(77)90347-3. [DOI] [PubMed] [Google Scholar]


