Abstract
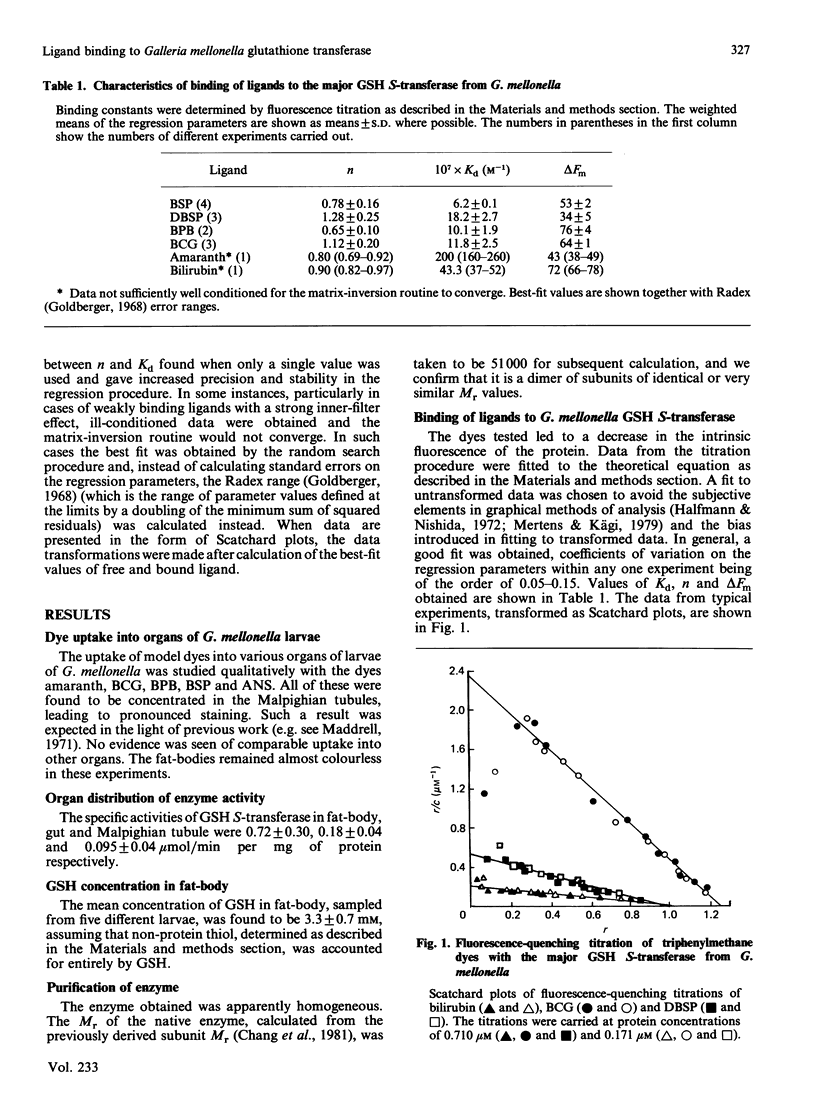
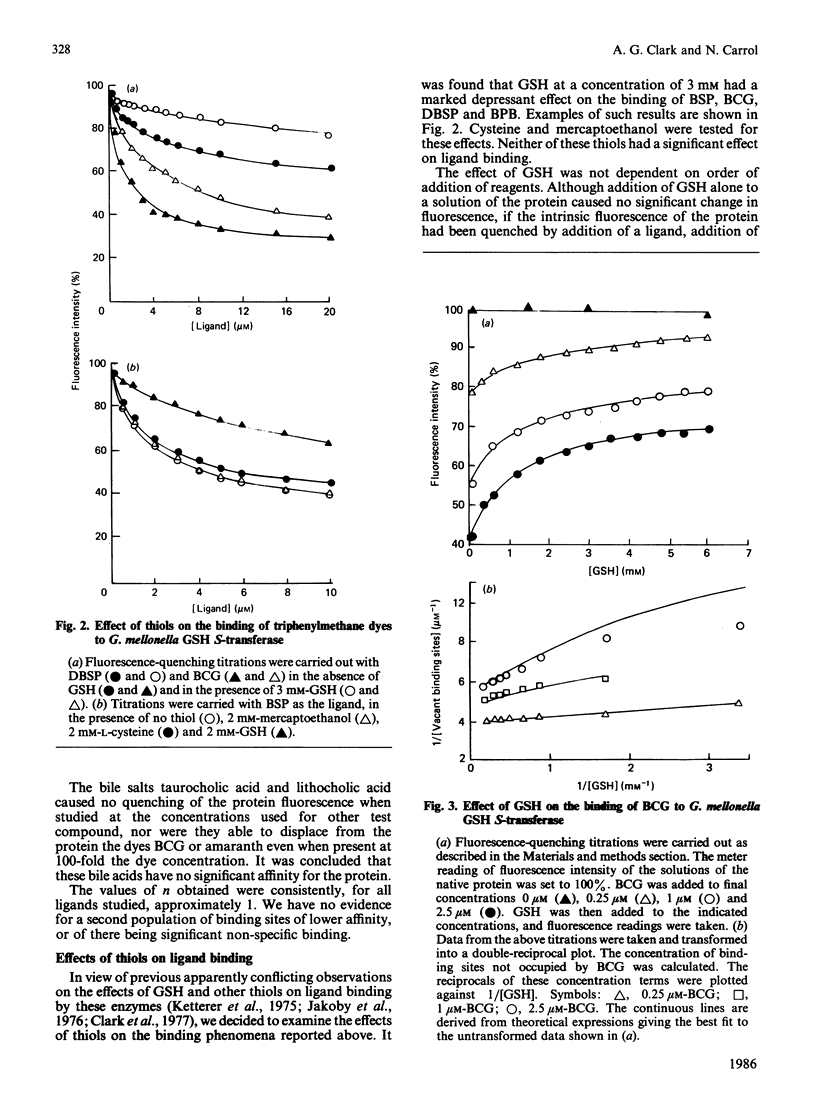
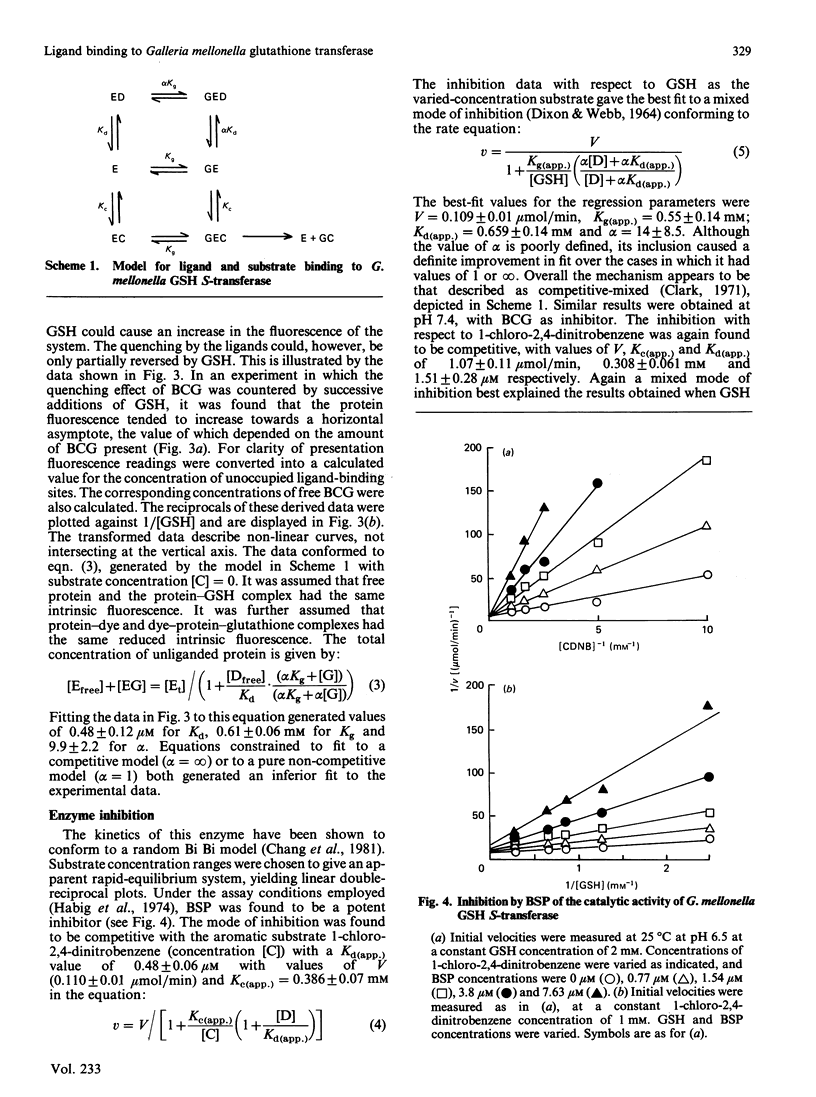
The major glutathione S-transferase from larvae of Galleria mellonella binds a number of synthetic triphenylmethane dyes with dissociation constants of the order of 10(-6) M or less. The organ distribution of the enzyme activity does not parallel the uptake of such dyes by the insect's organs in vivo. The affinity of the protein for such dyes is decreased by about an order of magnitude by the presence of glutathione in normal physiological concentration. This appears to be the cause of this protein's lack of efficacy as a 'ligandin' in vivo. The dyes appear to be acting as ineffective substrate analogues, binding at the catalytic site and impeding, in a reciprocal fashion, the binding of glutathione. Fluorescence-quenching titration and kinetic experiments together indicate the existence of a single ligand-binding and catalytic site per dimeric enzyme molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava M. M., Listowsky I., Arias I. M. Ligandin. Bilirubin binding and glutathione-S-transferase activity are independent processes. J Biol Chem. 1978 Jun 25;253(12):4112–4115. [PubMed] [Google Scholar]
- Boyer T. D., Vessey D. A., Holcomb C., Saley N. Studies of the relationship between the catalytic activity and binding of non-substrate ligands by the glutathione S-transferases. Biochem J. 1984 Jan 1;217(1):179–185. doi: 10.1042/bj2170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne T., Tipping E., Ketterer B. The binding and catalytic activities of forms of ligandin after modification of its thiol groups. Biochem J. 1979 Feb 1;177(2):433–439. doi: 10.1042/bj1770433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Darby F. J., Smith J. N. Species differences in the inhibition of glutathione S-aryltransferase by phthaleins and dicarboxylic acids. Biochem J. 1967 Apr;103(1):49–54. doi: 10.1042/bj1030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G., Letoa M., Ting W. S. The purification by affinity chromatography of a glutathione S-transferase from larvae of Galleria mellonella. Life Sci. 1977 Jan 1;20(1):141–147. doi: 10.1016/0024-3205(77)90140-0. [DOI] [PubMed] [Google Scholar]
- Clark A. G. Reversible inhibition in bimolecular rapid equilibrium random order enzyme systems. The effect of substrate-substrate and inhibitor-substrate interactions. Biochem J. 1970 May;117(5):997–1003. doi: 10.1042/bj1170997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Smith J. N., Turbert H. Comparative detoxication. 10. The enzymic conjugation of chloro compounds with glutathione in locusts and other insects. Biochem J. 1964 Mar;90(3):457–464. doi: 10.1042/bj0900457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Halfman C. J., Nishida T. Method for measuring the binding of small molecules to proteins from binding-induced alterations of physical-chemical properties. Biochemistry. 1972 Aug 29;11(18):3493–3498. doi: 10.1021/bi00768a025. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Chalmers J. Bile acid inhibition of basic and neutral glutathione S-transferases in rat liver. Biochem J. 1983 Dec 1;215(3):581–588. doi: 10.1042/bj2150581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagt D. L., Wilson S. P., Dean V. L., Simons P. C. Bilirubin binding to rat liver ligandins (glutathione S-transferases A and B). Relationship between bilirubin binding and transferase activity. J Biol Chem. 1982 Feb 25;257(4):1997–2001. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Meuwissen J., Beale D. Ligandin. Biochem Soc Trans. 1975;3(5):626–630. doi: 10.1042/bst0030626. [DOI] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mertens M. L., Kägi J. H. A graphical correction procedure for inner filter effect in fluorescence quenching titrations. Anal Biochem. 1979 Jul 15;96(2):448–455. doi: 10.1016/0003-2697(79)90605-5. [DOI] [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Organic anion-binding protein in rat liver: drug induction and its physiologic consequence. Proc Natl Acad Sci U S A. 1969 Sep;64(1):168–170. doi: 10.1073/pnas.64.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Kaplowitz N. Binding of glutathione by rat liver cytosol. Pharmacology. 1984;28(2):61–66. doi: 10.1159/000137945. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]


