Abstract
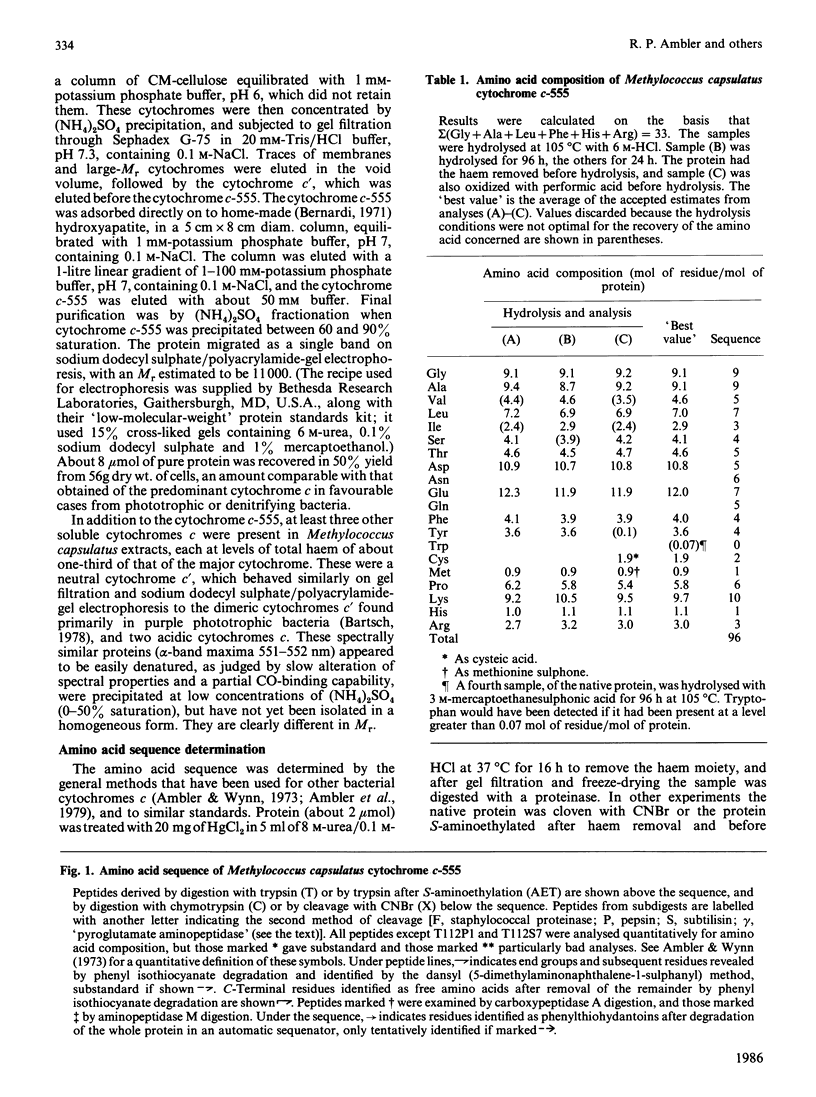
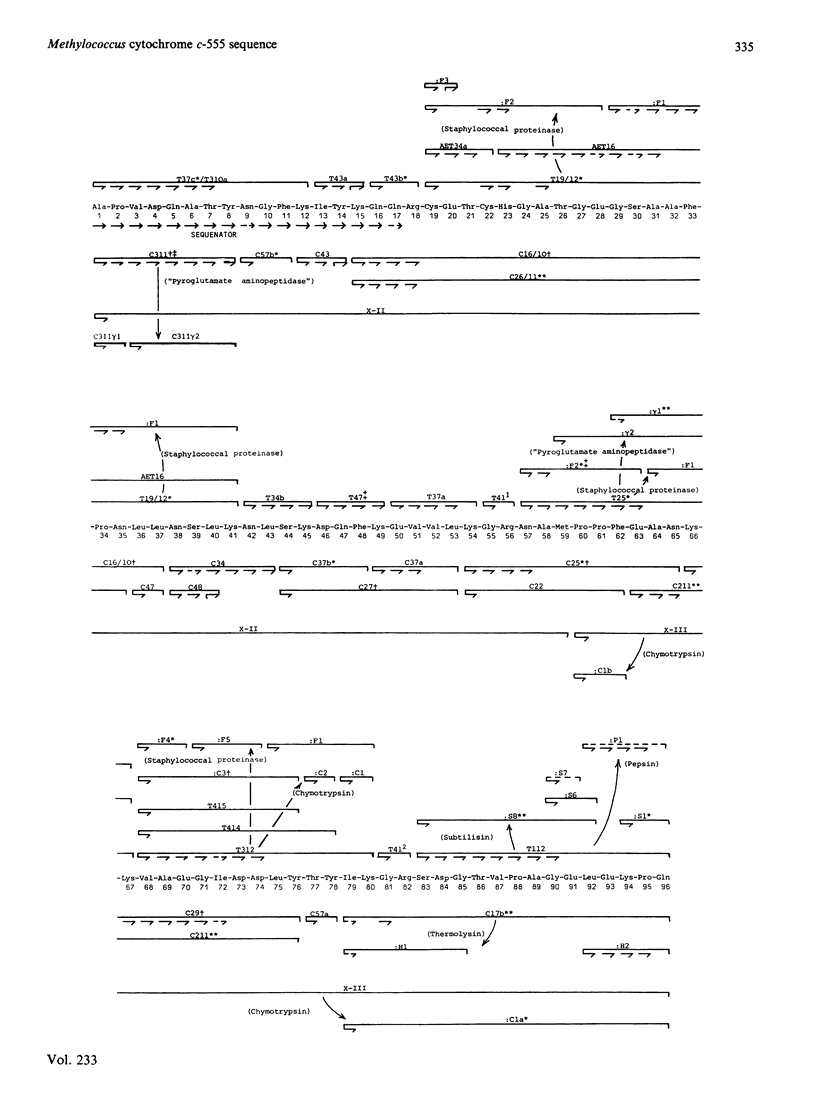
The amino acid sequence of the cytochrome c-555 from the obligate methanotroph Methylococcus capsulatus strain Bath (N.C.I.B. 11132) was determined. It is a single polypeptide chain of 96 residues, binding a haem group through the cysteine residues at positions 19 and 22, and the only methionine residue is a position 59. The sequence does not closely resemble that of any other cytochrome c that has yet been characterized. Detailed evidence for the amino acid sequence of the protein has been deposited as Supplementary Publication SUP 50131 (12 pages) at the British Library Lending Division, Boston Spa, West Yorkshire LS23 7BQ, U.K., from whom copies are available on prepayment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Bartsch R. G. Amino acid sequence similarity between cytochrome f from a blue-green bacterium and algal chloroplasts. Nature. 1975 Jan 24;253(5489):285–288. doi: 10.1038/253285a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Meyer T. E., Bartsch R. G., Kamen M. D. The amino acid sequence of cytochrome c' from the purple sulphur bacterium Chromatium vinosum. Biochem J. 1979 Mar 1;177(3):819–823. doi: 10.1042/bj1770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Kamen M. D. Primary structure determination of two cytochromes c2: close similarity to functionally unrelated mitochondrial cytochrome C. Proc Natl Acad Sci U S A. 1976 Feb;73(2):472–475. doi: 10.1073/pnas.73.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Trudinger P. A., Kamen M. D. The amino acid sequence of the cytochrome c-554(547) from the chemolithotrophic bacterium Thiobacillus neapolitanus. Biochem J. 1985 May 1;227(3):1009–1013. doi: 10.1042/bj2271009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Purification and properties of 3-hexulose phosphate synthase and phospho-3-hexuloisomerase from Methylococcus capsulatus. Biochem J. 1974 Dec;144(3):477–486. doi: 10.1042/bj1440477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. E., Kamen M. D. New perspectives on c-type cytochromes. Adv Protein Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]


