Abstract
Congestive heart failure (CHF) is a complex clinical syndrome that significantly impacts patient outcomes, especially in critically ill patients admitted to intensive care units (ICUs). The aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AST/ALT), has also been reported as a risk factor of cardiovascular diseases. However, few studies investigated the correlations between the AST/ALT ratio and ICU mortality in critically ill patients with CHF. This study investigates the association between the baseline AST/ALT ratio measured within the first 24 h of ICU admission and 28-day ICU all-cause mortality in critically ill patients with CHF. This retrospective cohort study included 4869 critically ill patients with CHF from the eICU Collaborative Research Database. Patients were categorized into tertiles based on their AST/ALT ratio: Tertile 1 (0.13–0.97), Tertile 2 (0.97–1.50), and Tertile 3 (1.50–5.89). Univariate and multivariate Cox proportional hazards regression models were used to evaluate the association between the AST/ALT ratio and 28-day ICU all-cause mortality. Nonlinear threshold effects and subgroup analyses were conducted to assess the robustness of the findings. Kaplan-Meier survival curves were generated to compare survival probabilities across tertiles. Participants with higher AST/ALT ratios were older, had higher illness severity, and experienced worse clinical outcomes. In univariate analysis, the AST/ALT ratio was significantly associated with 28-day ICU mortality (HR: 1.24, 95% CI 1.13–1.37, P < 0.0001). This association remained significant in the fully adjusted multivariate model. The highest tertile of AST/ALT ratio was associated with a significantly higher risk of mortality compared to the lowest tertile across all models (HR: 1.48, 95% CI 1.07–2.03, P = 0.0162 in Model 4). A nonlinear relationship was observed, with a threshold identified at an AST/ALT ratio of 2.08. Below this turning point, the association remained strong (HR: 1.47, 95% CI 1.13–1.91, P = 0.0036), while above it, the association was no longer significant. Subgroup analyses revealed no significant interactions, indicating that the association between AST/ALT ratio and mortality was consistent across various patient characteristics. Survival analysis showed that patients in the highest tertile had the poorest survival outcomes (P < 0.0001). An elevated AST/ALT ratio within the first 24 h of ICU admission is independently associated with increased 28-day ICU all-cause mortality in critically ill patients with CHF.
Keywords: Congestive heart failure (CHF), AST/ALT ratio, All-cause mortality, Intensive care unit (ICU)
Subject terms: Heart failure, Outcomes research
Introduction
Congestive heart failure (CHF) is a complex clinical syndrome in which the heart is unable to pump enough blood to meet the body’s needs, resulting in significant morbidity and mortality on a global scale, especially in critically ill patients who are admitted to intensive care units (ICUs)1. CHF often represents the end stage of various cardiovascular diseases (CVD), characterized by impaired cardiac function, neuroendocrine system activation, and abnormal distribution of peripheral blood flow. Despite advances in medical treatment, CHF continues to be a substantial healthcare challenge, with persistently high rates of mortality and frequent hospital re-admissions that impose a heavy burden on both patients and healthcare systems2. Early identification and accurate diagnosis of CHF are essential for improving patient outcomes, yet evaluating mortality in patients with CHF remains challenging3. Identifying high-risk patients who are prone to death during ICU is essential for developing targeted prevention strategies for this specific group.
Liver enzymes, particularly alanine aminotransferase (ALT) and aspartate aminotransferase (AST), have gained recognition as important markers for assessing liver damage4,5. While ALT is predominantly located in the liver, AST is presented in both the liver and myocardial tissues. The AST/ALT ratio, first introduced by De Ritis in 19576, is a well-established indicator of liver function and has demonstrated prognostic significance in CVD and other clinical contexts. Elevated AST/ALT ratio has been associated with poorer outcomes in patients suffering from heart failure7,8, cardiac arrest9, acute myocardial infarction10,11, hypertension12 and sepsis13. Furthermore, Yokoyama et al. reported that the AST/ALT ratio can predict both all-cause mortality and cardiovascular mortality in the general population14. In addition, Ewid et al. identified 0.9 as the optimal predictive cut-off value for the AST/ALT ratio in assessing the functional severity of CHF with reduced left ventricular ejection15.
However, to our knowledge, there has been no research specifically investigating the relationship between the AST/ALT ratio and all-cause mortality following ICU admission in critically ill patients with CHF. Therefore, this study aims to explore the association between the AST/ALT ratio and 28-day ICU all-cause mortality in this patient population.
Methods
Data source
The data for this study were derived from the eICU Collaborative Research Database (eICU-CRD), a large-scale, multi-center ICU database developed by the Laboratory for Computational Physiology at the Massachusetts Institute of Technology (MIT) in collaboration with Philips Healthcare. The eICU-CRD contains detailed information on more than 200,000 ICU admissions from 335 ICUs at 208 hospitals in the United States, collected between 2014 and 2015. This database includes high-resolution data such as vital signs, severity of illness scores, laboratory test results, diagnostic information, and treatment protocols.
The eICU-CRD complies with the Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor provisions, ensuring that all data is de-identified, eliminating the need for individual patient consent. The use of this database for research purposes was approved by the MIT Institutional Review Board (IRB), with a waiver of informed consent due to the retrospective nature of the study, the lack of direct patient intervention, and the adherence to secure data management practices that meet Safe Harbor standards. All experimental protocols were approved by the MIT IRB.
Access to the eICU-CRD is granted upon successful completion of the Collaborative Institutional Training Initiative (CITI) Data or Specimens Only Research course and certification. Once registered, researchers can download and use the data for their analyses. Corresponding author (Ping Jin) obtained access and was responsible for data extraction (certification number: 62661740). All procedures in this study were conducted in accordance with the ethical guidelines of the Declaration of Helsinki and followed the STROBE reporting standards.
Study population
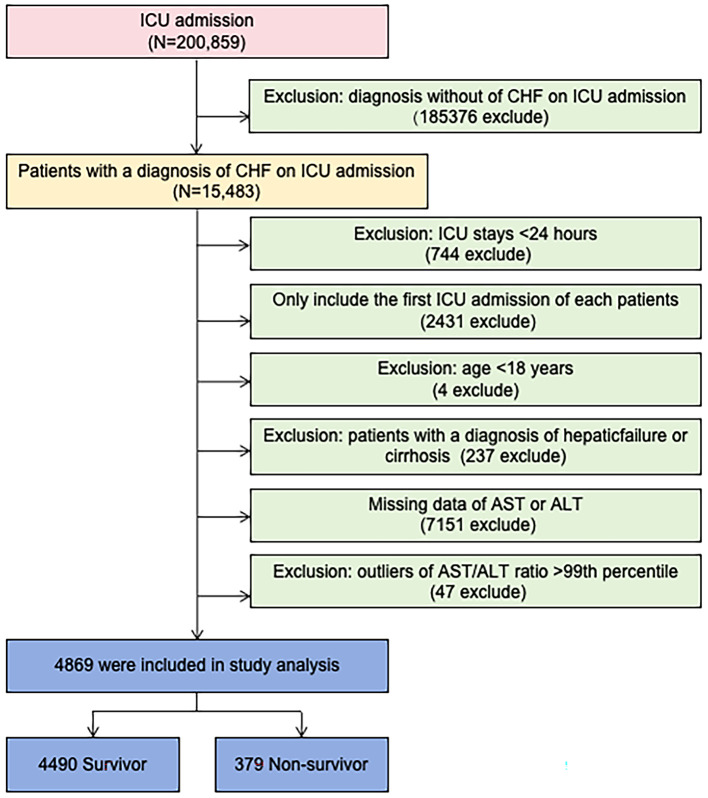
This study included patients admitted to the ICU with a diagnosis of congestive heart failure (CHF) based on the ICD-9 code in the eICU-CRD. The following exclusion criteria were applied: (1) ICU stay < 24 h, (2) not first ICU admission; (3) age < 18 years old; (4) patients with a diagnosis of hepatic failure or cirrhosis, which could affect the levels of AST and/or ALT; (5) missing data for AST or ALT; (6) outliers with AST/ALT levels above the 99th percentile. A total of 4,869 patients were included in the final study cohort. Of these, 379 patients did not survive, resulting in a 28-day ICU all-cause mortality rate of approximately 7.8%. The study flowchart is shown in Fig. 1.
Fig. 1.
Flow chart of study population. ICU intensive care unit. CHF congestive heart failure.
Variables
All relevant data for participants within the first 24 h of ICU admission were retrieved from the eICU-CRD using Structured Query Language (SQL). The physiological parameters, such as temperature (°C), respiratory rate (bpm), heart rate (HR, bpm) and mean arterial pressure (MAP, mmHg) were obtained from the apacheApsVar table. Baseline demographic information, including as age, gender, ethnicity and body mass index (BMI, kg/m2), was drawn from the tables of patient and apachePatientResult tables. Comorbidities including sepsis, chronic obstructive pulmonary disease (COPD), diabetes mellitus, acute myocardial infarction (AMI) and cardiac arrhythmias, were identified through the APACHE IV score. Sepsis was defined according to the Sepsis-3 criteria, which requires suspected or documented infection along with an acute rise of 2 or more points in the Sequential Organ Failure Assessment (SOFA) score from baseline16, as recorded in the Acute Physiology and Chronic Health Evaluation (APACHE) IV dataset17. Severity of illness was assessing using the Glasgow Coma Scale (GCS) score, Acute Physiology Score III and Apache IV score. Albumin(g/dL) was extracted as part of the laboratory data due to its role as a marker of nutritional status and its potential influence on liver function tests18. Hypoalbuminemia is known to be associated with worse outcomes in critically ill patients, particularly those with CHF19. Other laboratory results for creatinine (g/dL), while blood cells (WBC, k/mcl), red blood cells (RBC, k/mcl), hemoglobin (g/dL), platelets (k/mcl), aspartate aminotransferase (AST, U/L), and alanine aminotransferase (ALT, U/L) were obtained from the laboratory tables and represented the baseline which were recorded as the first measurement within 24 h after ICU admission. The AST/ALT ratio was calculated by dividing AST by ALT. The outcome of this study was all-cause mortality during ICU within 28 days after ICU admission among critically ill patients with CHF.
Statistical analysis
Continuous variables are described as mean ± standard deviation (SD). Categorical data are presented as frequencies and percentages. The difference according to the tertiles of the AST/ALT ratio was compared using one-way analysis of variance (ANOVA) for continuous data and chi-squared test for categorical variables (Table 1). We performed univariate (Table 2) and multivariate (Table 3) Cox proportional hazards regression models to investigate association between AST/ALT ratio and 28-day ICU all-cause mortality, and the results are presented as hazard ratios (HRs) and their 95% confidence intervals (95%CIs). Adjusted confounders were selected based on the results of univariate analysis and literature reports. After considering the clinical significance, we adjusted for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology Score III, Apache IV score and WBC. We used a generalized additive model (GAM) to investigate the dose-response relationship between the AST/ALT ratio and 28-day ICU all-cause mortality (Fig. 2). Two-piecewise linear regression models were used to test the threshold saturation effect of AST/ALT ratio on mortality. Exploratory analysis was used to determine the turning point of the AST/ALT ratio, and the point with the maximum likelihood of the model was selected. We also performed a likelihood ratio test to compare a linear regression model with a two-piecewise linear model. Stratified analysis and interaction tests were performed to determine whether the effect of AST/ALT ratio differed among subgroups, and the results are presented in the form of a forest plot (Fig. 3). Kaplan-Meier survival curves and the log-rank test were used to describe the survival distribution (Fig. 4). All reported P-values are two-tailed, and a P-value less than 5% is considered statistically significant. All data were analyzed using R software (v4.2.0) and Empower Stats (http://www.empowerstats.com, X&Y solutions, Inc. Boston MA).
Table 1.
Baseline characteristics and 28-day all-cause mortality according to the tertiles of the AST/ALT ratio.
| Characteristics | AST/ALT ratio | P value | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| 0.13–0.97 | 0.97–1.50 | 1.50–5.89 | ||
| n = 1622 | n = 1596 | n = 1651 | ||
| Demographics | ||||
| Age (years) | 68.10 ± 14.31 | 70.51 ± 13.59 | 70.66 ± 13.90 | < 0.001 |
| Gender | 0.001 | |||
| Male | 684 (42.17%) | 751 (47.06%) | 795 (48.15%) | |
| Female | 938 (57.83%) | 845 (52.94%) | 856 (51.85%) | |
| Ethnicity | 0.593 | |||
| Caucasian | 1209 (74.54%) | 1165 (72.99%) | 1223 (74.08%) | |
| Others | 413 (25.46%) | 431 (27.01%) | 428 (25.92%) | |
| BMI | 32.09 ± 10.39 | 30.84 ± 9.71 | 29.99 ± 9.04 | < 0.001 |
| Vital signs | ||||
| Respiratory rate (bpm) | 28.16 ± 13.75 | 28.61 ± 14.07 | 28.65 ± 14.65 | 0.560 |
| Heart rate (/min) | 99.62 ± 30.70 | 98.87 ± 30.87 | 102.05 ± 29.99 | 0.008 |
| MAP (mmHg) | 87.64 ± 41.79 | 85.26 ± 42.94 | 81.15 ± 42.82 | < 0.001 |
| Severity of illness | ||||
| GCS score | 13.69 ± 2.68 | 13.01 ± 3.36 | 12.74 ± 3.58 | < 0.001 |
| Acute Physiology Score III | 44.99 ± 20.22 | 50.19 ± 23.49 | 56.20 ± 26.11 | < 0.001 |
| Apache IV score | 58.83 ± 21.67 | 64.95 ± 24.56 | 70.93 ± 25.95 | < 0.001 |
| Comorbidities | ||||
| Sepsis | 203 (12.52%) | 235 (14.72%) | 314 (19.02%) | < 0.001 |
| COPD | 363 (22.38%) | 318 (19.92%) | 285 (17.26%) | 0.001 |
| Diabetes Mellitus | 397 (24.48%) | 376 (23.56%) | 364 (22.05%) | 0.252 |
| AMI | 103 (6.35%) | 121 (7.58%) | 223 (13.51%) | < 0.001 |
| Cardiac arrhythmias | 521 (32.12%) | 526 (32.96%) | 567 (34.34%) | 0.394 |
| Laboratory data | ||||
| Albumin (g/dL) | 2.99 ± 0.52 | 3.00 ± 0.59 | 2.87 ± 0.62 | < 0.001 |
| Lactate (mmol/L) | 1.90 ± 1.41 | 2.31 ± 2.17 | 2.86 ± 2.70 | < 0.001 |
| Creatinine (mg/dL) | 1.91 ± 1.79 | 2.09 ± 1.88 | 2.17 ± 1.78 | < 0.001 |
| cTn-I (ng/mL) | 0.67 ± 2.32 | 1.11 ± 2.91 | 7.24 ± 26.12 | < 0.001 |
| Total cholesterol (mg/dL) | 140.39 ± 40.50 | 141.92 ± 51.51 | 134.13 ± 44.90 | 0.138 |
| Triglycerides (mg/dL) | 115.34 ± 69.86 | 110.42 ± 61.62 | 111.09 ± 70.18 | 0.663 |
| HDL-C (mg/dL) | 40.18 ± 15.39 | 40.76 ± 15.71 | 38.38 ± 16.02 | 0.207 |
| LDL-C (mg/dL) | 76.72 ± 33.96 | 78.66 ± 42.06 | 76.42 ± 35.63 | 0.802 |
| WBC (k/mcl) | 10.64 ± 4.93 | 11.25 ± 2.24 | 10.72 ± 2.26 | < 0.001 |
| RBC (k/mcl) | 3.83 ± 0.77 | 3.80 ± 0.78 | 3.67 ± 0.78 | < 0.001 |
| Hemoglobin (g/dL) | 11.16 ± 2.27 | 11.04 ± 2.24 | 10.72 ± 2.26 | < 0.001 |
| Platelets (k/mcl) | 207.95 ± 92.53 | 207.07 ± 95.04 | 199.69 ± 96.07 | 0.027 |
| BNP (pg/mL) | 3377.42 ± 8218.08 | 3320.44 ± 8734.28 | 3840.76 ± 7407.87 | 0.007 |
| AST (U/L) | 67.23 ± 207.40 | 142.98 ± 643.80 | 260.25 ± 1079.01 | < 0.001 |
| ALT (U/L) | 99.19 ± 276.38 | 117.99 ± 516.85 | 121.66 ± 542.20 | < 0.001 |
| AST/ALT | 0.71 ± 0.18 | 1.21 ± 0.15 | 2.26 ± 0.85 | < 0.001 |
Data are expressed as the mean ± SD, median(interqurtile range), or number(percentage). Among the 4869 patients, the amount of missing values for the covariates were 120 (2.46%) for BMI, 21 (0.43%) for respiratory rate, 12 (0.25%) for heart rate, 17 (0.35%) for MAP, 60 (1.23%) for GCS score, 587 (12.1%) for Acute Physiology score III, 587 (12.1%) for Apache IV score, 54 (1.11%) for albumin, 32 (0.66%) for creatinine, 304(6.24%) for WBC, 231(4.74%) for RBC, 201(4.31%) for hemoglobin, 219 (4.50%) for platelets.
BMI body mass index, MAP mean artery pressure, GCS Glasgow Coma Scale, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, WBC white blood cells, RBC red blood cells, AST aspartate aminotransferase, ALT alanine aminotransferase.
Table 2.
The results of univariate Cox proportional hazards regression for 28-day all-cause mortaility after ICU admission.
| Exposure | Statistics | HR (95% CI) | P value |
|---|---|---|---|
| Age (years) | 69.76 ± 13.99 | 1.021 (1.013, 1.029) | < 0.0001 |
| Gender | |||
| Male | 2230 (45.80%) | Ref | 0.585 |
| Female | 2639 (54.20%) | 1.059 (0.863, 1.299) | |
| Ethnicity | 0.001 | ||
| Caucasian | 1272 (26.12%) | Ref | |
| Others | 3597 (73.88%) | 1.673 (1.281, 2.185) | |
| BMI (kg/m2) | 30.97 ± 9.76 | 0.982 (0.971, 0.993) | 0.001 |
| Respiratory rate (bpm) | 28.47 ± 14.16 | 1.009 (1.002, 1.016) | 0.009 |
| Heart rate (/min) | 100.20 ± 30.54 | 1.006 (1.002, 1.009) | 0.001 |
| MAP (mmHg) | 84.66 ± 42.59 | 0.997 (0.994, 0.999) | 0.018 |
| GCS score | 13.14 ± 3.25 | 0.934 (0.912, 0.956) | < 0.0001 |
| Acute Physiology Score III | 50.56 ± 23.89 | 1.018 (1.014, 1.021) | < 0.0001 |
| Apache IV score | 65.01 ± 24.65 | 1.019 (1.016, 1.023) | < 0.0001 |
| Sepsis | |||
| No | 4117 (84.56%) | Ref | 0.022 |
| Yes | 752 (15.44%) | 1.321 (1.041, 1.677) | |
| COPD | |||
| No | 3903 (80.16%) | Ref | 0.031 |
| Yes | 966 (19.84%) | 0.743 (0.567, 0.973) | |
| AMI | |||
| No | 4422 (90.82%) | Ref | 0.001 |
| Yes | 447 (9.18%) | 1.612 (1.219, 2.132) | |
| Cardiac arrhythmias | |||
| No | 3255 (66.85%) | Ref | 0.184 |
| Yes | 1614 (33.15%) | 1.148 (0.937, 1.407) | |
| Creatinine (mg/dL) | 2.06 ± 1.82 | 0.996 (0.939, 1.057) | 0.906 |
| WBC (k/mcl) | 11.41 ± 5.76 | 1.050 (1.035, 1.064) | < 0.0001 |
| RBC (k/mcl) | 3.76 ± 0.78 | 0.994 (0.875, 1.130) | 0.931 |
| Hemoglobin (g/dL) | 10.97 ± 2.27 | 1.020 (0.977, 1.066) | 0.370 |
| Platelets (k/mcl) | 204.85 ± 94.62 | 0.999 (0.998, 1.000) | 0.248 |
| AST (10U/L) | 157.51 ± 742.37 | 1.001 (1.000, 1.001) | 0.038 |
| ALT (10U/L) | 112.97 ± 461.20 | 1.001 (1.000, 1.002) | 0.037 |
| AST/ALT | 1.40 ± 0.83 | 1.244 (1.127, 1.374) | < 0.0001 |
BMI body mass index, MAP mean artery pressure, GCS Glasgow Coma Scale, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, WBC white blood cells, RBC red blood cells, AST aspartate aminotransferase, ALT alanine aminotransferase. HR hazard ratios, CI confidence interval.
Table 3.
Multivariate Cox proportional hazard regression analysis for association between AST/ALT ratio and 28-day all-cause mortality after ICU admission in different models.
| Exposure | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| AST/ALT | 1.24 (1.13, 1.37) | < 0.0001 | 1.28 (1.16, 1.42) | < 0.0001 | 1.25 (1.12, 1.39) | < 0.0001 | 1.18 (1.04, 1.34) | 0.0091 |
| AST/ALT tertile | ||||||||
| Low | ref | ref | ref | ref | ||||
| Middle | 1.33 (1.00, 1.76) | 0.0485 | 1.26 (0.95, 1.67) | 0.1055 | 1.22 (0.91, 1.62) | 0.1808 | 1.29 (0.92, 1.81) | 0.1347 |
| High | 1.74 (1.35, 2.26) | < 0.0001 | 1.70 (1.31, 2.21) | < 0.0001 | 1.60 (1.23, 2.09) | 0.0005 | 1.48 (1.07, 2.03) | 0.0162 |
| P for trend | 1.32 (1.16, 1.50) | < 0.0001 | 1.31 (1.15, 1.49) | < 0.0001 | 1.27 (1.12, 1.45) | 0.0003 | 1.20 (1.03, 1.40) | 0.0169 |
Data were presented as HR (95%CI) P value. Model 1: no adjustment for model variables. Model 2: adjust for gender, age and ethnicity. Model 3: adjust for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias. Model 4: adjust for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology score III, Apache IV score and WBC.
BMI body mass index, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, MAP mean artery pressure, GCS Glasgow Coma Scale, WBC white blood cells. HR hazard ratios, CI confidence interval.
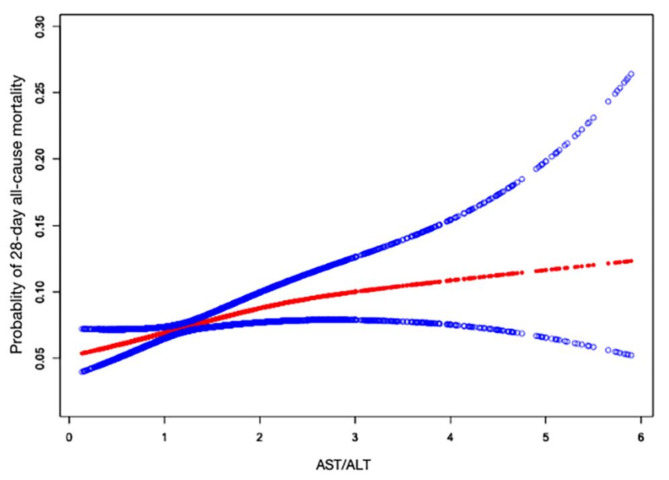
Fig. 2.
Associations between the AST/ALT ratio and 28-day all-cause mortality after ICU admission in critically ill patients with CHF. A nonlinear association between the AST/ALT ratio and 28-day all-cause mortality was found in a generalized additive model (GAM). Adjusted for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology Score III, Apache IV score and WBC. BMI body mass index, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, MAP mean artery pressure, GCS Glasgow Coma Scale, WBC white blood cells. The red lines represent the estimated values, and the blue lines represent their corresponding 95% confidence intervals.
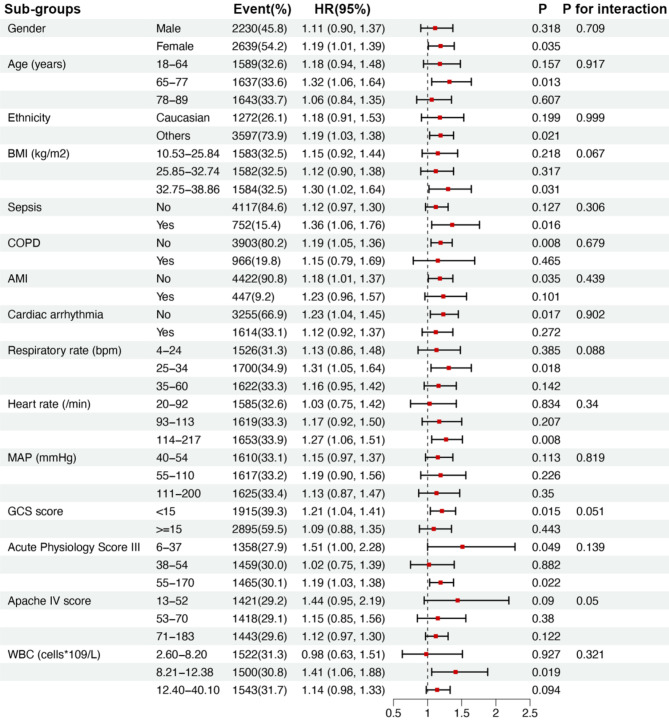
Fig. 3.
Subgroups analysis on the association between AST/ALT ratio and 28-day all-cause mortality after ICU admission with CHF. Except for the stratification component, each stratification was adjusted for all factors in Models 4 (adjust for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology Score III, Apache IV score and WBC). BMI body mass index, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, MAP mean artery pressure, GCS Glasgow Coma Scale, WBC white blood cells. HR hazard ratios, CI confidence intervals.
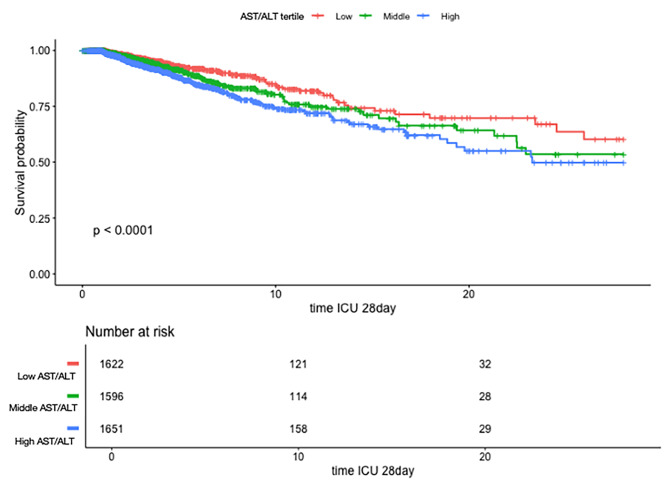
Fig. 4.
Kaplan–Meier survival curves of critically ill patients with CHF after ICU admission with different AST/ALT ratios. The range of AST/ALT ratio are as follows: Low 0.13–0.97, Middle 0.97–1.50, High 1.50–5.89.
Results
Basic characteristics
A total of 4869 critically ill patients with CHF were analyzed. Participants were categorized into three tertiles based on the AST/ALT ratio: Tertile 1 (0.13–0.97), Tertile 2 (0.97–1.50), and Tertile 3 (1.50–5.89). As the AST/ALT ratio increased, participants were found to be older, with a higher proportion of males. BMI decreased significantly across the tertiles. While no significant differences were observed in respiratory rates, heart rates were higher in Tertile 3, and MAP decreased with increasing AST/ALT ratios. Illness severity also increased across tertiles, with Tertile 3 patients having lower GCS scores and higher Acute Physiology Score III and APACHE IV scores, indicating more severe conditions. Sepsis and AMI were more prevalent in Tertile 3, while COPD was less common. Laboratory findings showed that patients in Tertile 3 had lower albumin levels, higher creatinine levels, and worse hemoglobin and RBC counts. AST and ALT levels rose significantly across tertiles, with a corresponding increase in the AST/ALT ratio.
Univariate Cox proportional hazards regression
The univariate analysis revealed several factors significantly associated with 28-day ICU mortality among patients with CHF. Older age was strongly correlated with increased risk (HR: 1.02, 95% CI 1.01–1.03, P < 0.0001). Ethnicity also showed a significant impact, with non-Caucasians having a higher risk of mortality compared to Caucasians (HR: 1.67, 95% CI 1.28–2.19, P = 0.001). A higher BMI was associated with a reduced risk (HR: 0.98, 95% CI 0.97–0.99, P = 0.001). Vital signs, including respiratory rate (HR: 1.01, P = 0.009) and heart rate (HR: 1.01, P = 0.001), were positively associated with mortality risk, while MAP showed a modest but significant association (HR: 1.00, P = 0.018). The severity of illness indicators all indicated that higher severity was linked to increased mortality risk. Sepsis (HR: 1.32, P = 0.022) and AMI (HR: 1.61, P = 0.001) were also significantly associated with higher mortality, while COPD was linked to a reduced risk (HR: 0.74, P = 0.031). Elevated WBC (HR: 1.05, P < 0.0001), AST (HR: 1.00, P = 0.038), ALT (HR: 1.00, P = 0.037), and the AST/ALT ratio (HR: 1.24, P < 0.0001) were associated with higher mortality. In contrast, creatinine, RBC, hemoglobin, and platelet levels did not show significant associations with mortality in the univariate analysis.
Multivariate Cox proportional hazards regression in different models
We developed 4 models to evaluate the independent association between AST/ALT ratio and 28-day ICU all-cause mortality. In Model 1, which no confounders were adjusted, higher AST/ALT ratios were significantly associated with increased mortality risk (HR: 1.24, 95% CI 1.13–1.37, P < 0.0001). This association remained robust across the other models, with Model 4 (the most fully adjusted model) showing a still significant but slightly attenuated relationship (HR: 1.18, 95% CI 1.04–1.34, P = 0.0091). When considering AST/ALT tertiles, patients in the highest tertile had a significantly higher risk of mortality compared to those in the lowest tertile across all models. In Model 1, the highest tertile was associated with a hazard ratio of 1.74 (95% CI 1.35–2.26, P < 0.0001). This association remained significant in Model 4, though the hazard ratio decreased to 1.48 (95% CI 1.07–2.03, P = 0.0162). The middle tertile, however, did not show a consistent significant association with mortality across the models. The trend analysis consistently demonstrated a positive association between increasing AST/ALT ratios and mortality risk across all models, with a significant P for trend value (P < 0.0001 in Model 1, and P = 0.0169 in Model 4).
Identification of nonlinear relationship between AST/ALT ratio and 28-day ICU all-cause mortality
We observed a nonlinear dose-response relationship between the AST/ALT ratio and mortality after adjusting for confounding factors (Fig. 2). Table 4 presents the threshold effect analysis examining the association between the AST/ALT ratio and 28-day ICU all-cause mortality, using two different models. In Model I, a linear relationship between the AST/ALT ratio and mortality was observed. Specifically, for each unit increase in the AST/ALT ratio, the HR for mortality was 1.18 (95% CI 1.04–1.34, P = 0.0091). Similarly, when analyzing per SD increase in the AST/ALT ratio, the HR was 1.15, also indicating a significant association. Model II explored the nonlinear threshold effect of the AST/ALT ratio, identifying a turning point (K) at a ratio of 2.08. Below this threshold (AST/ALT < 2.08), the relationship between the AST/ALT ratio and mortality remained strong and significant, with a HR of 1.47 (95% CI 1.13–1.91, P = 0.0036). However, above this threshold (AST/ALT > 2.08), the association became nonsignificant, with a HR of 0.98 (95% CI 0.77–1.25, P = 0.8847), suggesting that the increase in AST/ALT ratio beyond 2.08 no longer contributes to a higher mortality risk. The likelihood ratio test (LRT) confirmed a significant threshold effect in the model (P = 0.025), further supporting the existence of a nonlinear association between AST/ALT ratio and mortality.
Table 4.
Threshold effect analysis for association between AST/ALT ratio and 28-day all-cause mortality after ICU admission in different models.
| Models | AST/ALT per-unit increase | AST/ALT per-SD increase | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Model I | ||||
| One line effect | 1.18 (1.04, 1.34) | 0.0091 | 1.15 (1.03, 1.27) | 0.0091 |
| Model II | ||||
| Turning point(K) | 2.08 | 0.82 | ||
| AST/ALT < K | 1.47 (1.13, 1.91) | 0.0036 | 1.38 (1.11, 1.71) | 0.0036 |
| AST/ALT > K | 0.98 (0.77, 1.25) | 0.8847 | 0.99 (0.81, 1.20) | 0.8859 |
| P value for LRT test* | 0.025 | 0.025 | ||
Data were presented as HR (95% CI) P value. Model I: linear anaysis. Model II: non-linear analysis. Adjusted for gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology score III, Apache IV score and WBC.
BMI body mass index, COPD chronic obstructive pulmonary disease, AMI acute myocardial infarction, MAP mean artery pressure, GCS Glasgow Coma Scale, WBC white blood cells. HR hazard ratios, CI confidence interval. LRT logarithm likelihood ratio test.
*P < 0.05 indicates that Model II is significantly different from Model I.
Subgroup analysis
We used gender, age, ethnicity, BMI, sepsis, COPD, AMI, cardiac arrhythmias, respiratory rate, heart rate, MAP, GCS score, Acute Physiology Score III, Apache IV score and WBC as the stratification parameters to examine the robust of the association between the AST/ALT ratio and 28-day all-cause mortality after ICU admission. No interaction was discovered across the subgroups.
Kaplan–Meier survival analysis
Figure 4 displays Kaplan-Meier survival curves for critically ill patients with CHF after ICU admission, stratified by AST/ALT ratio tertiles. The three groups are as follows: Low (0.13–0.97), Middle (0.97–1.50), and High (n = 1651). The survival probability decreases over time in all groups, but the rate of decline varies significantly depending on the AST/ALT ratio. Patients in the High AST/ALT group (blue line) exhibit the steepest decline in survival probability, indicating the poorest outcomes. The log-rank test yielded a highly significant P-value (< 0.0001), suggesting that the differences in survival across the tertiles are statistically significant.
Discussion
In this study, we found a higher AST/ALT ratio was associated with a higher risk of 28-day ICU all-cause mortality in critically ill patients with CHF. Our findings revealed a significant and nonlinear association. To our knowledge, this is first time to report the association between AST/ALT ratio and ICU mortality in critically ill patients with CHF. This study contributes to the growing body of research investigating the clinical significance of the AST/ALT ratio in cardiovascular and systemic diseases, emphasizing its potential role in evaluating ICU mortality risk in critically ill patients with CHF.
The overall 28-day ICU all-cause mortality rate in our cohort was 7.8%. This mortality rate is relatively lower compared to some previous studies focused on critically ill patients with CHF, where reported mortality rates have ranged from 10 to 30%, depending on the severity of illness and comorbidities, specifically the different definition of mortality20,21. For instance, Wang et al. reported a 30-day in-hospital mortality rate of 15.96% among elderly heart failure patients using the eICU database22, and Chen et al. found an 11.6% in-hospital mortality rate in patients with heart failure after myocardial infarction using the MIMIC-IV database23. These studies involved populations with higher baseline risks, such as the elderly or post-myocardial infarction patients, which may explain the higher mortality rates. In contrast, Chang et al. reported a 28-day ICU mortality rate of 5.94% in patients with sepsis from the eICU database24, and Li et al. found a 30-day all-cause mortality rate of 6.2% in a critically ill population25, figures that align more closely with our findings. The lower mortality rate in our study could be attributed to the broader categorization of CHF patients in large databases like the eICU-CRD, where individuals with past episodes of cardiac decompensation may be labeled as CHF, even if they are not in active heart failure at the time of ICU admission. Additionally, differences in ICU management protocols and baseline illness severity could contribute to the variation in mortality rates across studies. Furthermore, the variation in mortality rates across studies highlights the complexity of predicting outcomes in critically ill CHF patients, underscoring the need for reliable biomarkers like the AST/ALT ratio to aid in early risk stratification and intervention26.
Our findings align with previous studies that have demonstrated an association between elevated AST/ALT ratios and worse clinical outcomes in cardiovascular diseases, including heart failure7,8, acute myocardial infarction10,11, and sepsis13. Similar to studies by Maeda et al.8 and Ewid et al.15, we found that a higher AST/ALT ratio was associated with increased mortality in patients with heart failure.
One of the unique contributions of the present investigation is the study population focus on critically ill patients with CHF. CHF is an end-stage manifestation of many cardiovascular diseases, and the prognosis of critically ill patients with CHF in the ICU is often worse. Our study goes beyond previous research by specifically examining critically ill patients with CHF in an ICU setting, a population where risk stratification is particularly critical due to their high mortality rates. The large sample size and multicenter nature of our study also strengthen the generalizability of our findings compared to smaller, single-center studies.
The identification of a nonlinear relationship between the AST/ALT ratio and mortality is another feature of this study. Unlike earlier studies8 that primarily focused on linear associations, we found that once the AST/ALT ratio exceeded a threshold of 2.08, its impact on mortality plateaued. This finding suggests that while moderate increases in the AST/ALT ratio may related to worse clinical conditions, extreme elevations may reflect other pathophysiological processes that are not directly related to mortality risk in patients with CHF15. Pathological conditions often result in tissue damage and abnormal physiological states, which lead to a more significant increase in AST compared to ALT, making the AST/ALT ratio a valuable marker. This is particularly prominent during the first 24 h of ICU admission, where the rise in the AST/ALT ratio is largely driven by elevated absolute AST levels. The AST/ALT ratio is widely believed to be closed related to liver function27, acute inflammation28,29, in some cases, systemic injury30. Since AST is present not only in the liver but also in the heart and muscles, elevated levels can point to both hepatic and extrahepatic damage31. In our study, this ratio likely represents these underlying mechanisms, particularly in patients with CHF, where liver congestion and cardiac stress are prevalent. The observed threshold effect at an AST/ALT ratio of 2.08 suggests that while moderate elevations may be associated with worse liver function, extreme elevations might reflect more complex multi-organ involvement, requiring careful interpretation. Besides, the threshold effect of could be influenced by the smaller number of patients with AST/ALT ratio exceeding 2.08, and future studies with larger sample sizes are needed to further validate this threshold.
The key strength of our study is the robust statistical methodology employed. We utilized multivariate Cox proportional hazards models to control for potential confounders32, as well as nonlinear modeling to capture threshold effects, which have not been extensively explored in previous studies21. Furthermore, our use of a large, multicenter ICU database enhances the external validity of our findings, making them applicable to a broad range of ICU settings and CHF populations33. This is in contrast to many earlier studies that relied on smaller, homogeneous cohorts, limiting their generalizability21. Another advantage of our study is the comprehensive subgroup analysis. We explored the consistency of the association between the AST/ALT ratio and mortality across various patient subgroups, including gender, age, ethnicity, and comorbidities. The lack of significant interactions across these subgroups suggests that the association between AST/ALT ratio and mortality of critically ill patients with CHF is robust.
Despite the overall alignment with existing literature, our findings also differ from some studies in key aspects. For example, while Maeda et al.7 identified a lower cut-off point (0.9) for the AST/ALT ratio as predictive of mortality in patients with heart failure, our study found that the mortality risk associated with the AST/ALT ratio was nonlinear, with a significant threshold effect at 2.08. This discrepancy could be due to differences in patient populations, as our study focused on critically ill ICU patients, whereas Maeda’s study included a broader spectrum of patients with heart failure. Critically ill patients may have more complex pathophysiological conditions, leading to different dynamics between liver enzymes and clinical outcomes34,35. Additionally, the differences in the study design, including variations in the measurement timing and patient follow-up, may also account for the observed discrepancies. In the design of this study, we excluded patients admitted to the ICU with cirrhosis or hepatic failure to minimize the other factors that could affect our primary outcome and to ensure the robustness of the results.
There were several limitations should be acknowledged. First, as an observational study, it cannot establish causality between the AST/ALT ratio and 28-day ICU all-cause mortality in critically ill patients with CHF. Second, unmeasured confounders are common problems in observational studies. Although key clinical factors were adjusted in multivariate analysis, not every factor associated with the AST/ALT ratio was included, as highlighted in previous reports. Third, while our study identified a significant nonlinear association between the AST/ALT ratio and mortality, larger sample size should be conducted to verify the threshold effects, and to clarify the exact pathophysiological pathways that drive this relationship. Lastly, while we focused on 28-day ICU all-cause mortality for critically ill patients with CHF after ICU admission, which is different from the general 28-day mortality. ICU mortality captures deaths occurring within the ICU setting but may underestimate overall mortality compared to hospital mortality, which accounts for deaths occurring after ICU discharge but before hospital discharge. Future studies should validate the findings in other critically ill populations and investigate the AST/ALT ratio’s role in different cardiovascular conditions.
Conclusion
In conclusion, our study demonstrates that elevated AST/ALT ratio is significantly associated with 28-day ICU all-cause mortality in critically ill patients with CHF. This association was nonlinear, when the AST/ALT ratio was below the turning point, the odds of 28-day ICU mortality increased; when the turning point was exceeded, the odds seemed no longer increased. Further research is needed to clarify the causal pathways and to investigate how this ratio can be effectively utilized in clinical practice to improve outcomes of critically ill patients with CHF.
Author contributions
Yitong Bian and Ping Jin wrote the manuscript; Huijuan Kou and Zhen Jia analyzed the data; Qing Cui, Juan Ma and Peng Wu: completed the validation; Xueping Ma supervised and revised this manuscript; Ping Jin designed the study.All authors reviewed the manuscript.
Funding
This research was funded by the Clinical Research Funds of the First Affiliated Hospital of Xi’an Jiaotong University (Grant No. XJTU1AF2022LSL-014), the Natural Science Foundation of Shaanxi Province, China (Grant No. 2022JQ-787), the Key Research and Development General Project in Shaanxi Province, China (Grant No. 2023-YBSF-605), the Natural Science Foundation of Shaanxi Province, China (Grant No. 2024JC-YBQN-0876) and the Natural Science Foundation of Ningxia, China (Grant No. 2023AAC02069).
Data availability
Data are fully available at https://eicu-crd.mit.edu/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueping Ma, Email: maxueping4033@126.com.
Ping Jin, Email: nxjinping1990@xjtu.edu.cn.
References
- 1.Savarese, G. et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 118, 3272–3287. 10.1093/cvr/cvac013 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Greene, S. J. et al. The vulnerable phase after hospitalization for heart failure. Nat. Rev. Cardiol. 12, 220–229. 10.1038/nrcardio.2015.14 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Tromp, J. et al. A systematic review and network Meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 10, 73–84. 10.1016/j.jchf.2021.09.004 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Mandato, C. & Vajro, P. Isolated aspartate aminotransferase elevation: is it liver disease or what else? Acta Paediatr. 111, 459–461. 10.1111/apa.16213 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Kulecka, M. et al. A heterozygous mutation in GOT1 is associated with familial macro-aspartate aminotransferase. J. Hepatol. 67, 1026–1030. 10.1016/j.jhep.2017.07.003 (2017). [DOI] [PubMed] [Google Scholar]
- 6.De Ritis, F., Coltorti, M. & Giusti, G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin. Chim. Acta. 2, 70–74. 10.1016/0009-8981(57)90027-x (1957). [DOI] [PubMed] [Google Scholar]
- 7.Maeda, D. et al. Relation of Aspartate Aminotransferase to alanine aminotransferase ratio to nutritional status and prognosis in patients with Acute Heart failure. Am. J. Cardiol. 139, 64–70. 10.1016/j.amjcard.2020.10.036 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Maeda, D. et al. Aspartate aminotransferase to alanine aminotransferase ratio is associated with frailty and mortality in older patients with heart failure. Sci. Rep. 11, 11957. 10.1038/s41598-021-91368-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, Z., Ma, G. & Chen, L. De-ritis ratio is Associated with Mortality after Cardiac arrest. Dis. Markers. 2020 (8826318). 10.1155/2020/8826318 (2020). [DOI] [PMC free article] [PubMed]
- 10.Ndrepepa, G., Holdenrieder, S. & Kastrati, A. Prognostic value of De Ritis ratio in patients with acute myocardial infarction. Clin. Chim. Acta. 535, 75–81. 10.1016/j.cca.2022.08.016 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Steininger, M. et al. De-ritis ratio improves long-term risk prediction after Acute myocardial infarction. J. Clin. Med. 7 10.3390/jcm7120474 (2018). [DOI] [PMC free article] [PubMed]
- 12.Liu, H. et al. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Med. (Baltim). 100, e26693. 10.1097/md.0000000000026693 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schupp, T. et al. Diagnostic and prognostic value of the AST/ALT ratio in patients with sepsis and septic shock. Scand. J. Gastroenterol. 58, 392–402. 10.1080/00365521.2022.2131331 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama, M. et al. Association of the aspartate Aminotransferase to Alanine Aminotransferase Ratio with BNP Level and Cardiovascular Mortality in the General Population: The Yamagata Study 10-Year Follow-Up. Dis Markers 4857917. 10.1155/2016/4857917 (2016). [DOI] [PMC free article] [PubMed]
- 15.Ewid, M. et al. AST/ALT ratio predicts the functional severity of chronic heart failure with reduced left ventricular ejection fraction. BMC Res. Notes. 13, 178. 10.1186/s13104-020-05031-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer, M. et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). Jama. 315, 801–810. 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman, J. E., Kramer, A. A., McNair, D. S. & Malila, F. M. Acute Physiology and Chronic Health evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit. Care Med. 34, 1297–1310. 10.1097/01.Ccm.0000215112.84523.F0 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Arroyo, V., García-Martinez, R. & Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 61, 396–407. 10.1016/j.jhep.2014.04.012 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Biancucci, M. et al. Hypoalbuminaemia and heart failure: a practical review of current evidence. Eur. J. Heart Fail. 10.1002/ejhf.3363 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Shah, K. S. et al. Heart failure with preserved, Borderline, and reduced ejection fraction: 5-Year outcomes. J. Am. Coll. Cardiol. 70, 2476–2486. 10.1016/j.jacc.2017.08.074 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Lee, D. S. et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. Jama. 290, 2581–2587. 10.1001/jama.290.19.2581 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Wang, Z. et al. A novel web-based calculator to predict 30-day all-cause in-hospital mortality for 7,202 elderly patients with heart failure in ICUs: a multicenter retrospective cohort study in the United States. Front. Med. (Lausanne). 10, 1237229. 10.3389/fmed.2023.1237229 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, Y. et al. Association between lactate/albumin ratio and mortality in patients with heart failure after myocardial infarction. ESC Heart Fail. 10, 1928–1936. 10.1002/ehf2.14359 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang, L., Chen, X. & Lian, C. The association between the non-HDL-cholesterol to HDL-cholesterol ratio and 28-day mortality in sepsis patients: a cohort study. Sci. Rep. 12, 3476. 10.1038/s41598-022-07459-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, S., Zhang, W. & Liu, H. Association between lipid levels and all-cause and cause-specific mortality in critically ill patients. Sci. Rep. 13, 5109. 10.1038/s41598-023-32209-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheorghiade, M. & Peterson, E. D. Improving postdischarge outcomes in patients hospitalized for acute heart failure syndromes. Jama. 305, 2456–2457. 10.1001/jama.2011.836 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Allen, L. A. et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 11, 170–177. 10.1093/eurjhf/hfn031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedé-Ubieto, R. et al. Abnormal liver function test in patients infected with coronavirus (SARS-CoV-2): a retrospective single-center study from Spain. J. Clin. Med. 10 10.3390/jcm10051039 (2021). [DOI] [PMC free article] [PubMed]
- 29.Sagaram, M. et al. One-month assessment of Th-cell axis related inflammatory cytokines, IL-17 and IL-22 and their role in alcohol-associated liver disease. Front. Immunol. 14, 1202267. 10.3389/fimmu.2023.1202267 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong, Y. et al. Association of IL-8 and CXCR2 with AST/ALT Ratio in Liver Abnormalities Screening during Oxidative Stress Injury Caused by VCM. J Toxicol 1951046, doi: (2024). 10.1155/2024/1951046 (2024). [DOI] [PMC free article] [PubMed]
- 31.Botros, M. & Sikaris, K. A. The De Ritis ratio: the test of time. Clin. Biochem. Rev. 34, 117–130 (2013). [PMC free article] [PubMed] [Google Scholar]
- 32.Austin, P. C., Lee, D. S. & Fine, J. P. Introduction to the Analysis of Survival Data in the Presence of competing risks. Circulation. 133, 601–609. 10.1161/circulationaha.115.017719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández, J. et al. Clinical course and short-term mortality of cirrhotic patients with infections other than spontaneous bacterial peritonitis. Liver Int. 37, 385–395. 10.1111/liv.13239 (2017). [DOI] [PubMed] [Google Scholar]
- 34.O’Leary, J. G. et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 67, 2367–2374. 10.1002/hep.29773 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Pollard, T. J. et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data. 5, 180178. 10.1038/sdata.2018.178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are fully available at https://eicu-crd.mit.edu/.