Abstract
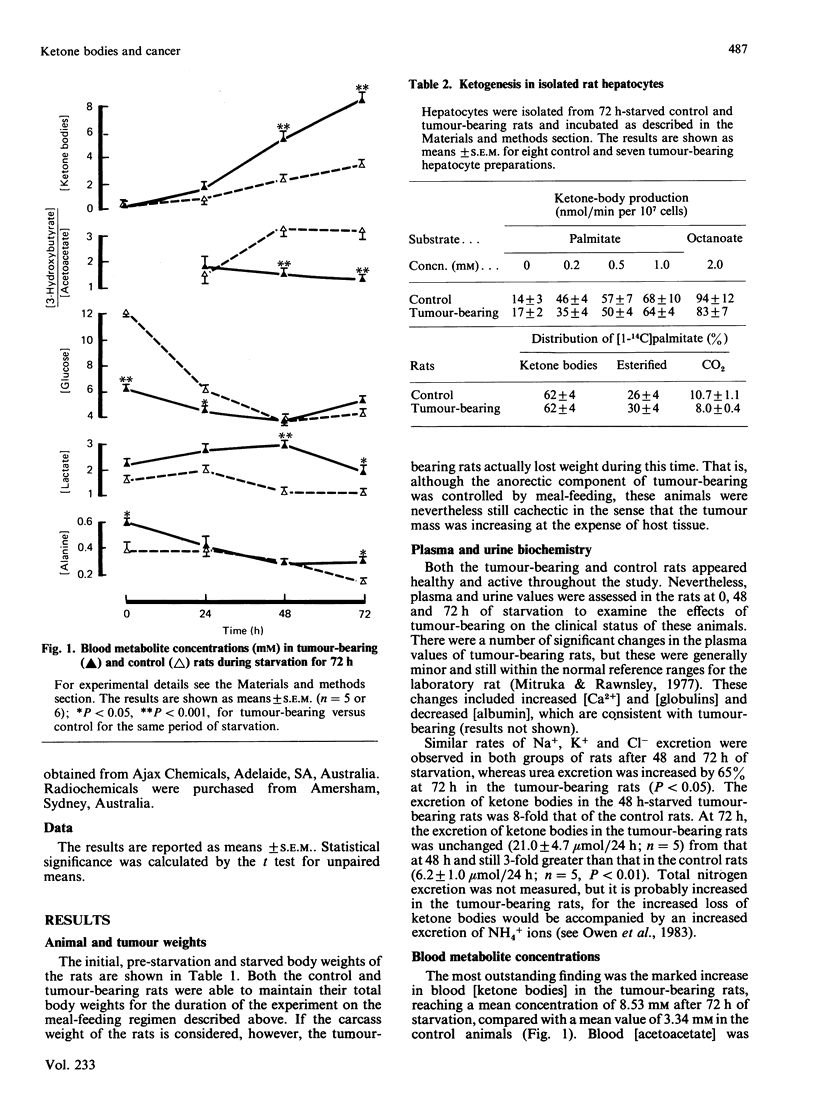
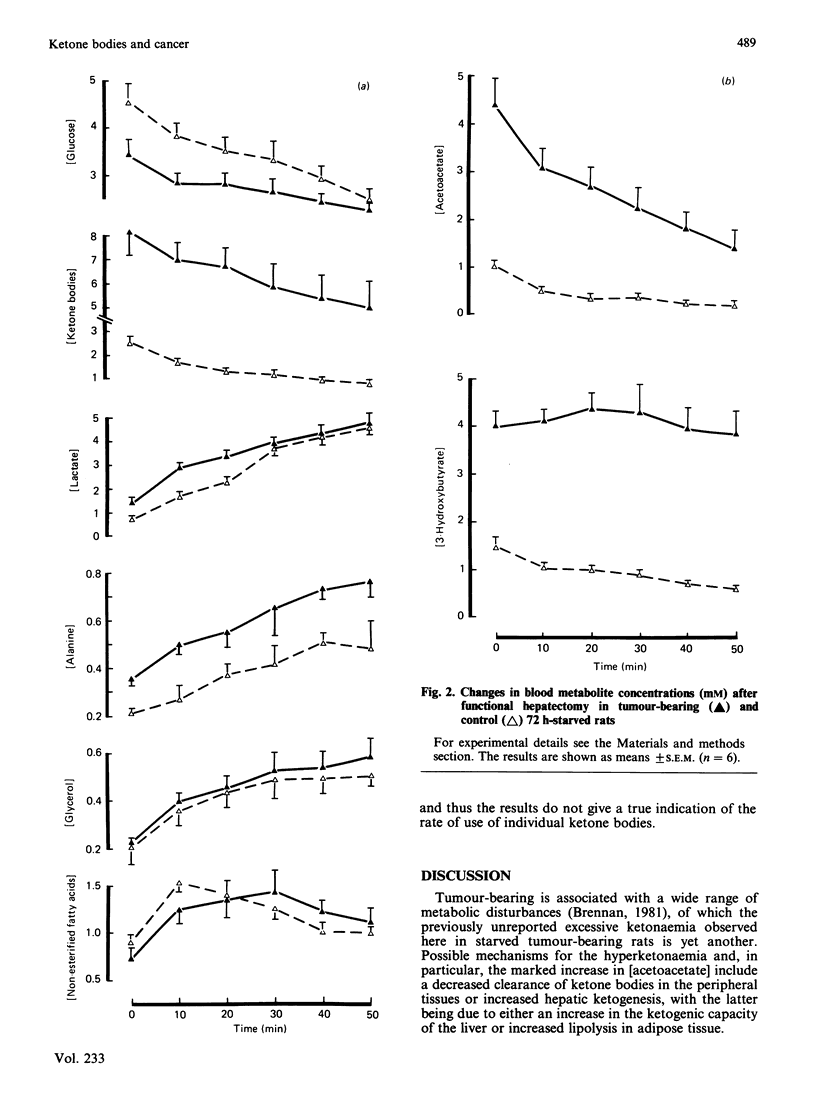
During starvation for 72 h, tumour-bearing rats showed accelerated ketonaemia and marked ketonuria. Total blood [ketone bodies] were 8.53 mM and 3.34 mM in tumour-bearing and control (non-tumour-bearing) rats respectively (P less than 0.001). The [3-hydroxybutyrate]/[acetoacetate] ratio was 1.3 in the tumour-bearing rats, compared with 3.2 in the controls at 72 h (P less than 0.001). Blood [glucose] and hepatic [glycogen] were lower at the start of starvation in tumour-bearing rats, whereas plasma [non-esterified fatty acids] were not increased above those in the control rats during starvation. After functional hepatectomy, blood [acetoacetate], but not [3-hydroxybutyrate], decreased rapidly in tumour-bearing rats, whereas both ketone bodies decreased, and at a slower rate, in the control rats. Blood [glucose] decreased more rapidly in the hepatectomized control rats. Hepatocytes prepared from 72 h-starved tumour-bearing and control rats showed similar rates of ketogenesis from palmitate, and the distribution of [1-14C] palmitate between oxidation (ketone bodies and CO2) and esterification was also unaffected by tumour-bearing, as was the rate of gluconeogenesis from lactate. The carcinoma itself showed rapid rates of glycolysis and a poor ability to metabolize ketone bodies in vitro. The results are consistent with the peripheral, normal, tissues in tumour-bearing rats having increased ketone-body and decreased glucose metabolic turnover rates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod L., Halter J. B., Cooper D. S., Aoki T. T., Roussell A. M., Bagshaw S. L. Hormone levels and fuel flow in patients with weight loss and lung cancer. Evidence for excessive metabolic expenditure and for an adaptive response mediated by a reduced level of 3,5,3'-triiodothyronine. Metabolism. 1983 Sep;32(9):924–937. doi: 10.1016/0026-0495(83)90208-1. [DOI] [PubMed] [Google Scholar]
- Balasse E. O. Kinetics of ketone body metabolism in fasting humans. Metabolism. 1979 Jan;28(1):41–50. doi: 10.1016/0026-0495(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Bates M. W. Effects of hydroxybutyrate infusion and insulin injection on ketone body turnover of rats. Am J Physiol. 1972 Feb;222(2):462–467. doi: 10.1152/ajplegacy.1972.222.2.462. [DOI] [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel W. R., Wannemacher R. W., Jr Gluconeogenesis, ureagenesis, and ketogenesis during sepsis. JPEN J Parenter Enteral Nutr. 1980 May-Jun;4(3):277–285. doi: 10.1177/014860718000400307. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Alberti K. G. The metabolic effects of sodium dichloroacetate in the starved rat. Biochem J. 1974 Aug;142(2):279–286. doi: 10.1042/bj1420279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal S. A. Stimulation of gluconeogenesis by palmitic acid in rat hepatocytes: evidence that this effect can be dissociated from the provision of reducing equivalents. Metabolism. 1983 Oct;32(10):971–976. doi: 10.1016/0026-0495(83)90137-3. [DOI] [PubMed] [Google Scholar]
- Brennan M. F. Total parenteral nutrition in the cancer patient. N Engl J Med. 1981 Aug 13;305(7):375–382. doi: 10.1056/NEJM198108133050705. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. Clin Endocrinol Metab. 1976 Jul;5(2):397–415. doi: 10.1016/s0300-595x(76)80028-x. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Ord V. A. Parenteral level of glucose intake on glucose homeostasis, tumor growth, gluconeogenesis, and body composition in normal and tumor-bearing rats. Cancer Res. 1983 Nov;43(11):5228–5234. [PubMed] [Google Scholar]
- Conyers R. A., Need A. G., Durbridge T., Harvey N. D., Potezny N., Rofe A. M. Cancer, ketosis and parenteral nutrition. Med J Aust. 1979 May 5;1(9):398–399. doi: 10.5694/j.1326-5377.1979.tb126984.x. [DOI] [PubMed] [Google Scholar]
- Conyers R. A., Need A. G., Rofe A. M., Potezny N., Kimber R. J. Nutrition and cancer. Br Med J. 1979 Apr 28;1(6171):1146–1146. doi: 10.1136/bmj.1.6171.1146-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaugre F., Buc H., Girard J., Leroux J. P. Role of the mitochondrial metabolism of pyruvate on the regulation of ketogenesis in rat hepatocytes. Metabolism. 1983 Jan;32(1):40–48. doi: 10.1016/0026-0495(83)90153-1. [DOI] [PubMed] [Google Scholar]
- Kralovic R. C., Zepp F. A., Cenedella R. J. Studies of the mechanism of carcass fat depletion in experimental cancer. Eur J Cancer. 1977 Oct;13(10):1071–1079. doi: 10.1016/0014-2964(77)90003-2. [DOI] [PubMed] [Google Scholar]
- Lanza-Jacoby S., Lansey S. C., Miller E. E., Cleary M. P. Sequential changes in the activities of lipoprotein lipase and lipogenic enzymes during tumor growth in rats. Cancer Res. 1984 Nov;44(11):5062–5067. [PubMed] [Google Scholar]
- MIDER G. B. Some aspects of nitrogen and energy metabolism in cancerous subjects: a review. Cancer Res. 1951 Nov;11(11):821–829. [PubMed] [Google Scholar]
- Magee B. A., Potezny N., Rofe A. M., Conyers R. A. The inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci. 1979 Oct;57(5):529–539. doi: 10.1038/icb.1979.54. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Miles J. M., Nissen S. L., Rizza R. A., Gerich J. E., Haymond M. W. Failure of infused beta-hydroxybutyrate to decrease proteolysis in man. Diabetes. 1983 Mar;32(3):197–205. doi: 10.2337/diab.32.3.197. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Caprio S., Reichard G. A., Jr, Mozzoli M. A., Boden G., Owen R. S. Ketosis of starvation: a revisit and new perspectives. Clin Endocrinol Metab. 1983 Jul;12(2):359–379. doi: 10.1016/s0300-595x(83)80046-2. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Baab P. J., Hawkins R. L., Ozand P. T. A double-isotope method for the measurement of ketone-body turnover in the rat. Effect of L-alanine. Biochem J. 1984 Apr 1;219(1):15–24. doi: 10.1042/bj2190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A. J., Wright P. D. Ketosis and nitrogen excretion in undernourished surgical patients. JPEN J Parenter Enteral Nutr. 1979 Sep-Oct;3(5):350–354. doi: 10.1177/014860717900300506. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., James H. M., Bais R., Edwards J. B., Conyers R. A. The production of (14C) oxalate during the metabolism of (14C) carbohydrates in isolated rat hepatocytes. Aust J Exp Biol Med Sci. 1980 Apr;58(2):103–116. doi: 10.1038/icb.1980.10. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Porter S. J., Bais R., Conyers R. A. The metabolic response of tumour-bearing mice to fasting. Br J Cancer. 1985 Oct;52(4):619–623. doi: 10.1038/bjc.1985.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofe A. M., Williamson D. H. Metabolic effects of vasopressin infusion in the starved rat. Reversal of ketonaemia. Biochem J. 1983 Apr 15;212(1):231–239. doi: 10.1042/bj2120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M. C., Sherwin R. S. Maternal ketosis and its effects on the fetus. Clin Endocrinol Metab. 1983 Jul;12(2):413–428. doi: 10.1016/s0300-595x(83)80049-8. [DOI] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Ketone body, glucose, lactic acid, and amino acid utilization by tumors in vivo in fasted rats. Cancer Res. 1983 Aug;43(8):3497–3503. [PubMed] [Google Scholar]
- Sauer L. A., Stayman J. W., 3rd, Dauchy R. T. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982 Oct;42(10):4090–4097. [PubMed] [Google Scholar]
- Schein P. S., Kisner D., Haller D., Blecher M., Hamosh M. Cachexia of malignancy: potential role of insulin in nutritional management. Cancer. 1979 May;43(5 Suppl):2070–2076. doi: 10.1002/1097-0142(197905)43:5+<2070::aid-cncr2820430715>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Grigor M. R., Thompson M. P. Glucose homeostasis in rats bearing a transplantable sarcoma. Cancer Res. 1980 May;40(5):1699–1706. [PubMed] [Google Scholar]
- Stein T. P. Cachexia, gluconeogenesis and progressive weight loss in cancer patients. J Theor Biol. 1978 Jul 6;73(1):51–59. doi: 10.1016/0022-5193(78)90179-0. [DOI] [PubMed] [Google Scholar]
- Thompson M. P., Koons J. E., Tan E. T., Grigor M. R. Modified lipoprotein lipase activities, rates of lipogenesis, and lipolysis as factors leading to lipid depletion in C57BL mice bearing the preputial gland tumor, ESR-586. Cancer Res. 1981 Aug;41(8):3228–3232. [PubMed] [Google Scholar]
- Tisdale M. J., Brennan R. A. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983 Feb;47(2):293–297. doi: 10.1038/bjc.1983.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]


