This systematic review and meta-analysis evaluates the association between meal timing strategies and anthropometric and metabolic outcomes.
Key Points
Question
What is the association between meal timing strategies and anthropometric and metabolic indicators?
Findings
In this systematic review and meta-analysis of 29 randomized clinical trials involving 2485 individuals, greater weight loss was achieved with time-restricted eating, lower meal frequency, and earlier caloric distribution in the day.
Meaning
This meta-analysis suggests that meal timing strategies, especially time-restricted eating, lower meal frequency, and consuming calories earlier in the day, may help an individual achieve greater weight loss when implemented for a minimum duration of 12 weeks, informing dietary recommendations for better weight management and improved metabolic health.
Abstract
Importance
Meal timing strategies, such as time-restricted eating (TRE), reducing meal frequency, or altering calorie distribution across the day, have gained interest for their potential to enhance weight loss and metabolic health, particularly in managing chronic diseases, yet their long-term benefits are not known.
Objective
To evaluate the association between meal timing strategies (≥12 weeks) and anthropometric and metabolic indicators.
Data Sources
Medline, Embase, CINAHL, and Cochrane CENTRAL were searched from inception to October 17, 2023.
Study Selection
Randomized clinical trials, regardless of language and publication date, involving adults 18 years and older, evaluating within-day meal timing patterns for 12 or more weeks, and reporting anthropometric measures were included. Studies were excluded if participants had eating disorders, prior significant weight change, underwent bariatric surgery, were pregnant, or if controlled variables differed between groups.
Data Extraction and Synthesis
Study quality was determined via Risk of Bias 2.0 tool. Data were extracted independently by multiple reviewers. Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used. Meta-analysis was performed using random-effects model on pooled continuous outcomes with 2 or more studies.
Main Outcome and Measures
Weight change in kilograms, reported as between-group mean difference with 95% CIs.
Results
Sixty-nine reports of 29 randomized clinical trials including 2485 individuals (1703 [69%] female; mean [SD] age, 44 [9.5] years; and mean [SD] body mass index, 33 [3.5]) were included. Study interventions included TRE (17 studies), meal frequency (8 studies), and calorie distribution (4 studies). There were some concerns of risk of bias for 7 studies and high concerns for 22 studies. Statistically significant weight change was observed in TRE when compared with control (–1.37 kg; 95% CI, –1.99 to –0.75 kg). Lower meal frequency and earlier caloric distribution were also both associated with greater change (–1.85 kg; 95% CI, –3.55 to –0.13 kg; and –1.75 kg; 95% CI, –2.37 to –1.13 kg, respectively).
Conclusions and Relevance
The findings of this meta-analysis suggest that TRE, lower meal frequency, and earlier caloric distribution in the day may reduce weight compared with standard care and/or nutritional advice; however, the effect sizes found were small and of uncertain clinical importance. High heterogeneity and risk of bias among included studies led to concerns about the certainty of the underpinning evidence. Further research, including trials with larger sample sizes, standardized interventions with prescribed or matched energy intake, and longer follow-up, are needed.
Introduction
One in 8 people are living with obesity and 43% are overweight.1 Overweight and obesity are associated with increased risk of type 2 diabetes, heart disease, cancers, and premature death.2 Dietary modification is a key element of obesity management and includes reducing calorie intake or altering macronutrient composition or dietary patterns.3,4,5 Long-term adherence is a major challenge for many dietary approaches to weight loss. While calorie reduction is fundamental for weight loss, recent interest has emerged in meal timing strategies (ie, temporal distribution of meals throughout the day), such as time-restricted eating (TRE), reducing meal frequency, and altering calorie distribution across the day, for their potential to provide an alternative strategy for individuals who find daily demands of counting calories in traditional continuous energy restriction approaches challenging.6,7,8,9,10,11,12
Many individuals eat for more than 14 hours a day and snack late at night, which can worsen glycemic control and increase the risk of type 2 diabetes.13 Therefore, intermittent fasting is a popular weight loss strategy. TRE, a form of intermittent fasting, involves fasting and eating within a 24-hour cycle, typically consolidating calorie intake to 6- to 10-hour periods during the active phase of the day, without necessarily altering diet quality and quantity.
Meal timing approaches (including TRE) might represent a promising, attractive, and easy-to-adapt strategy for the management of obesity and prevention of metabolic disorders. To date, research efforts including systematic reviews have focused on randomized clinical trials (RCTs) in adults with overweight or obesity with a primary focus on single meal timing approaches—reducing calories in evening meals, varying eating windows across the day, and follow-up durations (typically <12 weeks) limiting long-term efficacy of such interventions since short-term benefits are often not sustained in the long-term.3 Subsequent trials in diverse populations with longer follow-up have been published, showing mixed beneficial effects on weight loss and metabolic health.14,15,16,17,18,19,20,21 Therefore, the aim of this systematic review was to evaluate existing evidence and determine the long-term (beyond 12 weeks) association between meal timing strategies and anthropometric and metabolic outcomes in adults with or without metabolic disease.
Methods
This systematic review adhered to the Cochrane methods22 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.23 We prospectively registered the protocol with PROSPERO (CRD42023474391). The Bond University human research ethics committee deemed this study exempt from approval and the need for informed consent because it collected and synthesized publicly available nonidentifiable data from previously published studies.
Data Sources and Search Strategy
We searched Medline (via Ovid), Embase (via Elsevier), CINAHL (via EBSCO host), and Cochrane CENTRAL on October 17, 2023. We designed the search strategy using free text and key terms including meal frequency, meal timing, time-restricted eating, and intermittent fasting, with no language restrictions. We used systematic review accelerator tools24 to refine and translate the search for other databases (eTable 1 in Supplement 1). Forward-backward citation analysis was performed using SpiderCite.24 Duplicate citations were removed using Deduplicator24 and Covidence.22
Eligibility Criteria and Study Selection
We included RCTs involving adults aged 18 years and older with or without comorbidities and evaluated the association between temporal distribution of isocaloric meals throughout the biological day (including TRE, meal frequency, and calorie distribution) and weight or body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) over 12 or more weeks. Studies were excluded if they involved participants with eating disorders, who had underwent bariatric surgery, or who were pregnant. We excluded non-RCTs and observational studies. Two reviewers (A.E., N.C., V.L., and L.A.) independently screened titles and abstracts and full texts in duplicate using Covidence.22 Discrepancies were resolved through consensus.
Data Extraction
Two reviewers (A.E., N.C., and V.L.) extracted data independently using a prospectively developed data extraction template. Discrepancies were resolved through consensus or in consultation with a third reviewer (L.A.). Extracted data included: (1) study characteristics (eg, country and study design); (2) participant characteristics (eg, comorbidities and BMI); and (3) details of intervention(s) (eg, intensity and delivery). Primary outcomes were anthropometric measures (ie, body weight, BMI, and waist circumference). Secondary outcomes were metabolic (ie, glycated hemoglobin; HbA1c, fasting glucose, low-density lipoprotein cholesterol; LDL, blood pressure; BP, and energy intake).
For each outcome, we extracted first the within-group pre-post mean difference (MD) from baseline to the latest follow-up and SD. If not reported, baseline and latest follow-up measures were extracted, and the pre-post MD and SD were calculated.25 When SDs were not reported, we estimated SD from SEs, CIs, and P values.25 We converted median (IQR) to mean (SD).26 When data were not available, we extracted missing data from the studies’ other published reports. If still missing, we contacted authors for necessary data. If data were not received within 6 weeks from the initial request date, the data were not included.
Risk of Bias Assessment and Certainty of Evidence
Two reviewers (A.E., N.C., V.L., and L.A.) independently assessed the risk of bias in the effect of assignment of intervention (ie, intention-to-treat analysis) on primary and secondary outcomes for each included RCT using the Cochrane Risk of Bias 2 tool.27 Discrepancies were resolved through consensus. We assessed the risk of bias as low, some concerns, or high for each of the following domains: bias due to randomization, deviations from the intended intervention, missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results.
We rated the overall certainty of evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach in 5 domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias.28 Certainty of evidence was rated as high, moderate, low, or very low certainty. Disagreements were resolved with consensus and, as necessary, in consultation with a third reviewer (L.A.). We reported intervention effects using GRADE-recommended language.29
Statistical Analysis
Data Synthesis and Analysis
We performed meta-analyses when 2 or more studies reported data for the same outcome. We used an inverse-variance random effects model to calculate the pooled effect estimates using Review Manager RevMan version 5.4 (Cochrane Collaboration)30 and R version 4.3.1, meta package (R Project for Statistical Computing).
We presented pooled effect estimates as MDs with 95% CIs for continuous outcomes. All data analyzed were converted to conventional units.31 When trials had multiple treatment groups, we divided the number of participants in the placebo group by the number of treatment groups.
We assessed statistical heterogeneity using the Cochrane Q χ2 test and quantified using the I2 statistic, where lower than 25% indicated low heterogeneity, 25% to 50% indicated moderate heterogeneity, and greater than 50% indicated high heterogeneity. We assessed publication bias and/or small studies effect using visual inspection of a funnel plot when 10 or more RCTs were available within an analysis. Two-sided P values less than .05 were considered statistically significant.
Subgroups and Sensitivity Analysis
We conducted subgroup analyses by gender (≥80% women vs <80% women), obesity status (healthy, overweight, or obese BMI), comorbid conditions (healthy vs metabolic), intervention nature and intensity (eg, frequency of sessions), and follow-up duration (≥6 months vs <6 months). We conduced sensitivity analysis by risk of bias limiting to low-risk RCTs.
Results
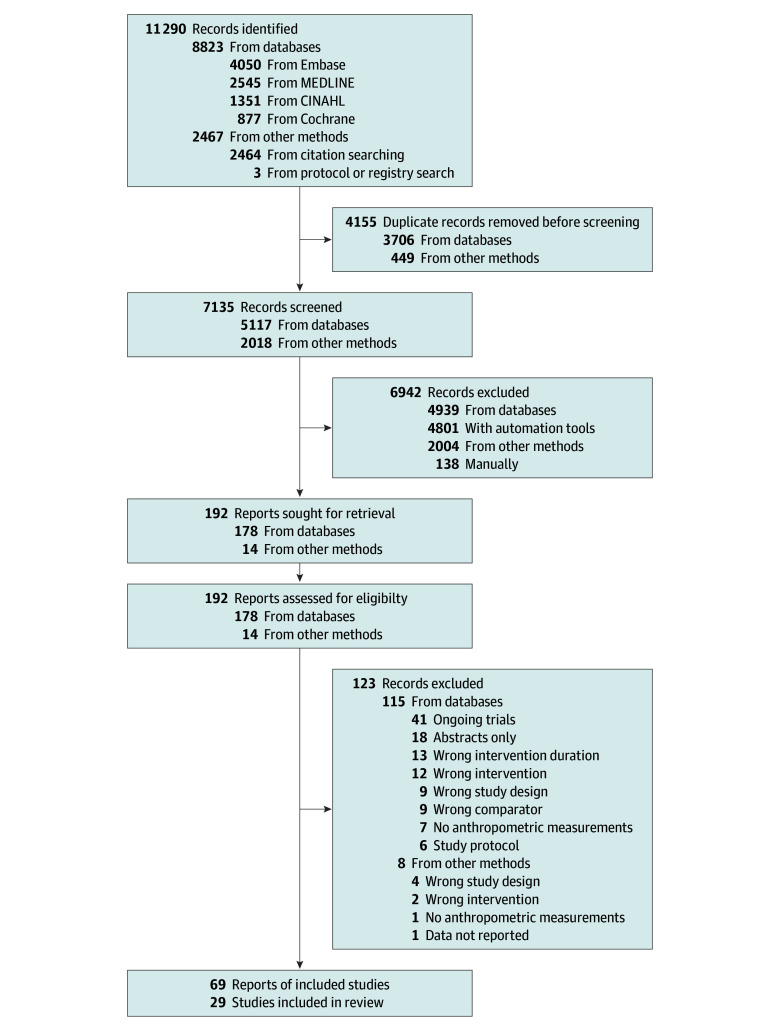
A total of 11 290 records were retrieved, of which 4155 were duplicates. A total of 7135 titles and abstracts were screened, and 192 potentially relevant full texts were screened for eligibility (Figure 1). Of those, we excluded 123 articles with reasons for exclusion recorded (eTable 2 in Supplement 1). Overall, we included 29 trials reported in 69 articles14,15,16,17,18,19,20,21,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52 (eTable 3 in Supplement 1), which reported on weight (26 articles),14,15,16,17,18,19,20,21,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,49,50,51 BMI (21 articles),14,15,16,17,19,21,32,33,34,35,38,41,42,43,44,45,46,47,49,50,52 lean and/or fat-free mass (13 articles),14,15,18,19,21,32,34,40,42,43,45,49,51 waist circumference (14 articles),14,15,16,17,19,21,35,38,40,41,43,44,47,49 HbA1c (19 articles),15,16,17,18,19,20,21,33,34,39,40,41,42,45,46,47,48,49,51 fasting glucose (24 articles),14,15,16,17,18,19,20,21,33,34,37,38,40,41,42,43,44,45,46,47,51,52 LDL (22 articles),14,15,16,17,18,19,20,21,33,34,37,38,40,41,42,43,44,45,46,47,51,52 systolic and diastolic BP (16 articles),15,18,19,20,21,34,35,37,38,40,42,43,44,45,46,49 and energy intake (13 articles).15,16,19,20,21,32,33,40,41,42,43,46,51
Figure 1. Flow Diagram of Included Randomized Clinical Trials.
Characteristics of Included Studies
The 29 included RCTs14,15,16,17,18,19,20,21,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52 enrolled a total of 2485 participants (a median [IQR] of 73 [49-110] per RCT), with a median (IQR) follow-up duration of 12 (12-26) weeks. Most study populations were of middle age (mean [SD] age 44 [9.5] years), predominantly female (1703 female [69%]); and overweight or obese (mean [SD] BMI 33 [3.5]). Most (27 trials [90%])14,15,16,17,18,19,20,21,32,33,34,35,36,37,38,39,40,42,43,44,45,46,49,50,51,52 of the included RCTs were parallel RCTs and one-third were conducted in the US (10 trials [34%]).15,19,32,34,36,40,45,46,50,51 Half of included RCTs (16 trials [55%])16,17,20,35,37,38,39,40,41,43,44,46,47,48,50,51 recruited participants from outpatient and/or community settings. Two-thirds of RCTs (22 trials [76%])14,15,16,17,20,21,32,33,34,35,36,37,38,40,41,42,43,44,45,48,49,52 enrolled healthy overweight or obese populations. Sixteen RCTs (55%)15,16,17,19,32,35,37,38,39,40,42,44,46,47,48,51 engaged clinicians who were specifically trained in nutrition or dietetics to deliver the interventions. The median (IQR) number of sessions required to administer the intervention in the included RCTs was 16 (10-19). Table 1 and eTable 4 in Supplement 1 provide details of included RCTs.
Table 1. Study Characteristics of Included Studies.
| Source, y, and country | Duration, RCT typea | Baseline participant characteristics | Intervention groups | Energy balance, macronutrient distribution and cointerventions | Outcomes measured | |||
|---|---|---|---|---|---|---|---|---|
| No. of participants (% female) | Age, mean (SD), y | Population health | BMI, mean (SD)b | |||||
| Time-restricted eating | ||||||||
| Che et al,33 2021 China | 12 wk, parallel | 120 (45.0) | C: 48.7 (9.5) I: 48.2 (9.3) |
T2D overweight and obese | C: 26.1 (2.1) I: 26.4 (2.0) |
C: Eat ad libitum per usual habits I: 10 h TRE (ad libitum), 8 am-6 pm |
No instruction provided on energy intake or macronutrient distribution | Weight, fasting glucose, HbA1c, LDL-C, energy intake, adherence |
| Chow et al,34 2020 US | 12 wk, parallel | 20 (85.0) | C: 44.2 (12.3) I: 46.5 (12.4) |
Healthy overweight and obese | C: 34.4 (7.8) I: 33.8 (7.6) |
C: Eat ad libitum per usual habits I: 8 h TRE (ad libitum during self-selected eating window) |
No instruction provided on energy intake or macronutrient distribution | Weight, FFM, lean mass, HbA1c, fasting glucose, LDL-C, BP, adherence, energy intakec |
| de Oliveira Maranhao Pureza et al,35 2021 Brazil | 52 wk, parallel | 58 (100.0) | C: 31.0 (7.1) I: 31.8 (6.9) |
Healthy obese women | C: 33.1 (3.6) I: 33.5 (4.5) |
C: Daily energy restriction I: 12 h TRE (self-selected eating window) |
ER = TEE – 500-1000 kcal/d Individualized meal plan based on usual diet. |
Weight, BMI, WC, BP, energy intake |
| Dhurandhar et al,36 2014 Denmark and US | 16 wk, parallel | 309 (75.7) | C: 42.1 (11.2) I: 42.0 (12.4) |
Healthy overweight and obese | Not stated | C: Usual eating habits; followed general good nutrition habits I: No energy intake before 11 am |
Each group provided with USDA pamphlet with instructions related to their specific intervention | Weight, adherence |
| Jamshed et al,40 2022 US | 14 wk, parallel | 90 (80.0) | C: 43.0 (11.0) I: 43.0 (10.0) |
Healthy obese | C: 39.2 (6.8) I: 40.1 (6.6) |
C: Self-selected ≥12 h eating window I: 8 h TRE (7 am-3 pm) |
ER = REE – 500 kcal/d Encouraged to increased exercise to 75-150 min/wk |
Weight, WC, FFM, fasting glucose, HbA1c, LDL-C, BP, adherence, energy intake, hunger and satiety (VAS)c |
| Kunduraci et al,42 2020 Turkey | 12 wk, parallel | 70 (51.4) | C: 48.7 (2.1) I: 47.4 (2.2) |
Metabolic syndrome overweight and obese | C: 32.8 (4.1) I: 36.5 (5.3) |
C: Daily energy restriction I: 8 h TRE (self-selected eating window) |
ER = −25% of habitual energy intake Personalized meal plan based on Turkey National Dietary Guidelines (Mediterranean diet) |
Weight, BMI, WC, FFM, BP, LDL-C, fasting glucose, HbA1c, energy intakec |
| Lin et al,15 2023 US | 52 wk, parallel | 60 (83.3) | C: 44.0 (13.0) I: 44.0 (12.0) |
Healthy obese | C: 38.0 (5.0) I: 37.0 (6.0) |
C: Maintained weight, physical activity habits and ≥10 h baseline eating window I: 8 h TRE (ad libitum 12-8 pm); at 26 wk, eating window widened to 10 h |
No instruction provided on energy intake or macronutrient distribution | Weight, BMI, WC, lean mass, fasting glucose, HbA1c, LDL-C, BP, adherence, energy intakec |
| Liu et al,43 2022 China | 52 wk, parallel | 139 (48.9) | C: 32.3 (8.8) I: 31.6 (9.3) |
Healthy overweight and obese | C: 31.3 (2.6) I: 31.8 (2.9) |
C: No time restriction I: 8 h TRE (8 am-4 pm) |
ER = 1500-1800 kcal/d for men and 1200-1500 kcal/d for women Macronutrient distribution (40%-55% carbohydrate, 15%-20% protein, 20%-30% fat) |
Weight, BMI, WC, lean mass, fasting glucose, LDL-C, BP, energy intake, adherencec |
| Lowe et al,45 2020 US | 12 wk, parallel | 116 (39.7) | C: 46.1 (10.3) I: 46.8 (10.8) |
Healthy overweight and obese | C: 32.6 (3.4) I: 32.9 (4.9) |
C: 3 meals daily (7-11 am, 11 am-3 pm, 4-10 pm); snacking between meals was permitted I: 8 h TRE (ad libitum, 12-8 pm) |
No instruction provided on energy intake or macronutrient distribution | Weight, BMI, WC, lean mass, fasting glucose, HbA1c, LDL-C, BP, adherence, energy intakec |
| Manoogian et al,46 2022 US | 12 wk, parallel | 137 (9.0) | C: 39.6 (9.4) I: 41.1 (8.7) |
Healthy fire fighters | C: 27.7 (3.9) I: 27.8 (3.6) |
C: No time restriction I: 10 h TRE (ad libitum during self-selected window) |
No ER Mediterranean diet (60% carbohydrates, 15% protein and 25% fat) |
Weight, BMI, HbA1c, fasting glucose, BP, LDL-C, energy intake, adherence |
| Montero et al,18 2023 Spain | 12 wk, parallel | 197 (50.0) | C: 46.7 (6.0) I: 46.8 (6.3) |
Overweight and obese with ≥1 cardiometabolic risk factor | C: 33.4 (3.6) I: 32.9 (3.3) |
C: Usual care I1: 8 h TRE (starting by 10:00 am) I2: 8 h TRE (starting by 1:00 pm) I3: 8 h TRE (self-selected eating window) |
No ER Education on Mediterranean diet |
Weight, lean mass, BP, fasting glucose, LDL-C, HbA1cc |
| Pavlou et al,19 2023 US | 26 wk, parallel | 75 (71.0) | C:54.0 (11.0) I:56.0 (13.0) |
Obese and T2D | C: 39.0 (7.0) I: 39.0 (9.0) |
C: Usual care I:8 h TRE (ad libitum 12-8 pm) |
No ER General healthy eating instructions |
Weight, HbA1c, lean mass, WC, BMI, BP, LCL-C, energy intake, adherencec |
| Philips et al,49 2021 Switzerland | 26 wk, parallel | 54 (NA) | C: 42.5 (14.0) I: 44.3 (12.8) |
Metabolic syndrome | C: 27.0 (4.0) I: 28.0 (4.1) |
C: Usual care I: 12 h TRE (ad libitum, self-selected eating window) |
No instruction provided on energy intake or macronutrient distribution | Weight, BMI, WC, BP, fasting glucose, HbA1c |
| Roman et al,50 2020 US | 26 wk, parallel | 24 (NA) | C and I: 41.6 (11.3) | Relapse Remitting Multiple Sclerosis | 25.1 (4.9) | C: Usual diet I: 8 h TRE (ad libitum, self-selected eating window) |
No instruction provided on energy intake or macronutrient distribution | Weight, BMI, adherence |
| Suthutvoravut et al,20 2023 Thailand | 12 wk, parallel | 46 (69.6) | C: 52.2 (7.9) I: 55.5 (7.2) | Prediabetes overweight and obese | C: 30.3 (3.2) I: 29.2 (2.9) |
C: Usual care I: 9 h TRE (ab libitum 8 am-5 pm) |
No instruction provided on energy intake or macronutrient distribution | Weight, fasting glucose, HbA1c, LDL-C, BP |
| Thomas et al,51 2022 US | 39 wk, parallel | 81 (85.2) | C: 37.8 (7.8) I: 38.3 (7.9) |
Healthy overweight and obese | C: 35.2 (4.7) I: 34.8 (6.4) |
C: No time restriction I: 10 h TRE (starting within 3 h of waking) |
ER = REE – 10% Encouraged to perform 150 min/wk of moderate activity |
Weight, BMI, FFM, fasting glucose, HbA1c, LDL-C, BP, adherence, energy intake, hunger and satiety (VAS and TFEQ)c |
| Wei et al,21 2023 China | 52 wk, parallel | 88 (44.3) | C: 31.7 (8.3) I: 32.3 (10.5) |
NAFLD obese | C: 32.2 (3.2) I: 32.2 (3.4) |
C: No time restriction I: 8 h TRE (8 am-4 pm) |
ER = 1500-1800 kcal/d for men and 1200-1500 kcal/d for women Macronutrient composition (40% to 55% carbohydrate, 15% to 20% protein, and 20% to 30% fat) |
Weight, BMI, WC, FFM, fasting glucose, HbA1c, LDL-C, BP, adherence, energy intakec |
| Meal frequency | ||||||||
| Bachman et al,32 2012 US | 26 wk, parallel | 51 (57.8) | I1: 51.8 (9.1) I2: 50.2 (10.8) |
Healthy overweight or obese | I1: 34.9 (4.3) I2: 36.1 (5.2) |
I1: 3Mdiet I2: Grazing group: ≥100kcal every 2-3 h (approximately 10 snacks) |
ER = 1200 kcal/d for participants ≤200 lbs 1500 kcal/d for participants >200 lbs Fat intake restricted to <30% energy Encouraged to increase physical activity to 200 min moderate intensity per wk |
BMI, FFM, energy intakec |
| Forslund et al,37 2008 Sweden | 52 wk, parallel | 140 (74.0) | I1: 38.7 (11.6) I2: 40.1 (11.5) |
Healthy obese | I1: 38.3 (5.3) I2: 38.4 (6.0) |
I1: 3Mdiet I2: 3 snacks plus 3 meals per day |
Energy restriction = TEE – 30% (minimum 1400 kcal/d) | Weight, BMI, fasting glucose, LDL-C, BP, energy intake |
| Grangeiro et al,14 2021 Brazil | 13 wk, parallel | 47 (100.0) | I1 [n = 19]: 29.05 (9.18) I2 [n = 21]: 30.33 (6.72) |
Healthy obese women | I1: 34.9 (1.6) I2: 35.2 (3.9) |
I1: 6Mdiet I2: 3Mdiet |
ER = TEE – 700 kcal Macronutrient distribution: 57% carbohydrates, 23% fat, 20% protein |
Weight, BMI, WC, FFM, fasting glucose, LDL-C, energy intake, adherencec |
| Jakubowicz et al,39 2019 Israel | 12 wk, parallel | 35 (61.0) | I1: 68 (8.6) I2: 69.5 (5.6) |
Insulin-treated T2D | I1: 32.1 (5) I2: 32.6 (5) |
I1:3Mdiet, large breakfast and smaller dinner I2: 6M diet (breakfast, lunch, dinner, and 3 snacks, even distribution) |
ER = REE – 500 kcal Macronutrient distribution: 40% carbohydrates, 35% fat, 25% protein |
Weight, fasting glucose, HbA1c, hunger (VAS) |
| Kahleova et al,41 2014 Czech Republic | 12 wk, crossover | 54 (46.0) | 59.4 (7.0) | T2D and overweight or obese | 32.6 (4.9) | I1: 6Mdiet I2: 2Mdiet (breakfast and lunch). |
ER = REE – 500 kcal Macronutrient distribution: 50%-55% carbohydrates, 20%-25% protein, <30% fat (<7% saturated fat) Meals provided for 50% of participants in each group |
Weight, BMI, WC, fasting glucose, HbA1c, LDL-C, energy intake |
| Papakonstantinou et al,47 2016 Greece | 12 wk, crossover | 40 (100.0) | 27.0 (1.0) | Women with PCOS | I1: 27.3 (1.0) I2: 27.2 (0.9) |
I1: 3Mdiet I2: 6Mdiet |
Energy maintenance Macronutrient distribution: 40% carbohydrates, 25% protein, 35% fat |
Weight, BMI, WC, HbA1c, fasting glucose, LDL-C, energy intake, hunger, satiety |
| Papakonstantinou et al,48 2018 Greece | 12 wk, crossover | 35 (57.1) | I1: 48.5 (3.2) I2: 52.1 (2.7) |
Prediabetes with overweight or obesity | I1: 32.6 (1.4) I2: 32.5 (1.2) |
I1: 3Mdiet I2: 6Mdiet |
Energy maintenance Macronutrient distribution: 45% carbohydrates, 20% protein, 35% fat |
Weight, BMI, WC, hunger, satiety, energy intake, HbA1c, fasting glucose, LDL-C |
| Papakonstantinou et al,48 2018 Greece | 12 wk, crossover | 12 (41.7) | I1: 52.1 (2.7) I2: 51.7 (3.5) |
Newly diagnosed treatment-naïve T2D with overweight or obesity | I1: 32.5 (1.2) I2: 32.2 (1.5) |
I1: 3Mdiet I2: 6Mdiet |
Energy maintenance Macronutrient distribution: 45% carbohydrates, 20% protein, 35% fat |
Weight, BMI, WC, hunger, satiety, energy intake, HbA1c, fasting glucose, LDL-C |
| Zargaran et al,52 2014 Iran | 12 wk, parallel | 90 (80.0) | NA (20-60 y) | Healthy overweight | C: 30.3 (4.7) I: 30.9 (5.2) |
C: Normal diet (most consisted of 3 meals and 2 snacks) I: 6Mdiet (iso-caloric meals) |
ER = TEE – 400 kcal | BMI, LDL-C |
| Calorie distribution | ||||||||
| Jakubowicz et al,38 2013 Israel | 12 wk, parallel | 93 (100.0) | I1: 45.1 (7.46) I2: 46.5 (6.86) |
Metabolic syndrome overweight and obese | I1: 32.3 (0.2) I2: 32.2 (0.2) |
I1: HCB (breakfast 700 kcal, lunch 500 kcal, dinner 200 kcal) I2: HCD (breakfast 200 kcal, lunch 500 kcal, dinner 700 kcal) |
ER = 1400 ± 25 kcal | Weight, BMI, WC, BP, LDL-C, fasting glucose, hunger, satiety, adherence |
| Lombardo et al,44 2014 Italy | 12 wk, parallel | 42 (100.0) | C: 43.0 (16.0) I1: 39.0 (17.0) |
Healthy overweight and obese | C: 35.1 (4.5) I1: 35.8 (5.2) |
C: Equal energy distribution (55% energy from breakfast, morning snack and lunch; 45% energy from afternoon snack and dinner) I: Front loading energy distribution (70% energy from breakfast, morning snack and lunch; 30% energy from afternoon snack and dinner) |
ER = TEE – 600 kcal Macronutrient composition: 16% protein, 25% fat, 59% carbohydrates |
Weight, BMI, WC, lean mass, fasting glucose, LDL-C, BP, energy intakec |
| Madjd et al,16 2016 Iran | 12 wk, parallel | 80 (100.0) | I1: 33.9 (7.3) I2: 33.3 (6.7) |
Healthy overweight and obese women | I1: 32.2 (2.2) I2: 32.1 (2.3) |
I1: Middle loading (energy distribution: 15% breakfast, 15% snacks, 50% lunch, 20% dinner) I2: Backloading (energy distribution: 15% breakfast, 15% snacks, 20% lunch, 50% dinner) |
ER (specifics unknown) Macronutrient composition: 17% protein, 23% fat (<10% saturated fat), 60% carbohydrate) Encouraged to increase physical activity to 60 min moderate activity 5 d/wk |
Weight, BMI, WC, LDL-C, fasting glucose, HbA1c, energy intake |
| Madjd et al,17 2021 Iran | 12 wk, parallel | 82 (100.0) | I1: 35.1 (7.4) I2: 34.9 (7.1) |
Healthy overweight and obese | I1: 32.7 (2) I2: 32.7 (2) |
I1: Early dinner eaten between 7-7:30 pm I2: Late dinner eaten between 10:30-11 pm |
ER = TEE – 500-1000 kcal Energy distribution: 15% breakfast, 15% snacks, 50% lunch, 20% dinner Macronutrient composition: 17% protein, 23% fat (<10% saturated fat), 60% carbohydrate Encouraged to increase physical activity to 60 min moderate activity 5 d/wk |
Weight, BMI, WC, fasting glucose, HbA1c, LDL-C, energy intake, adherence |
Abbreviations: 2Mdiet, 2 meals per day; 3Mdiet, 3 meals per day; 6Mdiet, 6 meals per day; BMI, body mass index; BP, blood pressure; C, control group; ER, energy restriction; FFM, fat-free mass; HbA1c, glycated hemoglobin; HCB, high-calorie breakfast; HCD, high-calorie dinner; I, intervention group; LDL-C, low-density lipoprotein cholesterol; NA, not available; NAFLD, nonalcoholic fatty liver disease; PCOS, polycystic ovary syndrome; RCT, randomized clinical trial; REE, resting energy expenditure; T2D, type 2 diabetes; TEE, total energy expenditure; TFEQ, Three Factor Eating Questionnaire; TRE, time-restricted eating; USDA, United States Department of Agriculture; VAS, visual analog scale; WC, waist circumference.
Follow-up duration in most studies was equal to intervention duration.
Calculated as weight in kilograms divided by height in meters squared.
Lean mass and FFM have both been reported as lean mass in this study.
Of 17 RCTs (59%)15,18,19,20,21,33,34,35,36,40,42,43,45,46,49,50,51 that evaluated TRE, 10 (59%)15,18,19,21,34,40,42,43,45,50 implemented an 8-hour feeding window, and 11 (65%)15,18,19,20,33,34,36,45,46,49,50 instructed all participants to eat freely (TRE plus ad libitum vs ad libitum). Eight RCTs (28%)14,32,37,39,41,47,48,52 evaluated meal frequency; two-thirds (5 trials [63%]14,37,39,47,48) compared 3 meals per day with 6 meals per day. Four RCTs (14%)16,17,38,44 compared calorie distribution across the biological day.
Risk of Bias
The overall risk of bias in the results of the effect of meal timing for the primary outcome (ie, weight measurement) was deemed high for two-thirds of included RCTs (22 trials [76%]),14,16,17,18,20,32,33,34,36,37,38,39,41,42,44,46,47,48,49,50,51,52 mostly because of bias in measurement of outcomes and missing outcome data. Seven RCTs15,19,21,35,40,43,45 were judged to have some concerns for overall risk of bias. None of the included RCTs judged low overall risk of bias (eFigures 1 and 2 in Supplement 1).
Main Findings
TRE
Anthropometric Measures
Overall, 17 RCTs15,18,19,20,21,33,34,35,36,40,42,43,45,46,49,50,51 (1527 participants) were included in the meta-analysis of the effect of TRE on weight change. TRE may reduce weight (MD, –1.37 kg; 95% CI, –1.99 to –0.75 kg; I2 = 73%; low-certainty evidence) (Figure 2 and Table 2). Substantial heterogeneity was partly explained by baseline BMI status (P for interaction = .02) and intervention intensity (P for interaction = .04). Participants with higher baseline BMI lost more weight than those with lower BMI (eFigure 3 in Supplement 1). Furthermore, RCTs with feeding times 8 hours or less per day were associated with greater weight loss (MD, –1.88 kg; 95% CI, –2.72 to –1.04 kg) compared with RCTs with feeding times more than 8 hours per day (MD, –0.71 kg; 95% CI, –1.42 to 0.00 kg), suggesting a dose-response association (eFigure 4 in Supplement 1).
Figure 2. Meta-Analysis of Difference in Mean Difference (95% CIs) for the Effect of Meal Timing Interventions on Weight, Grouped by the Nature of the Meal Timing Intervention.
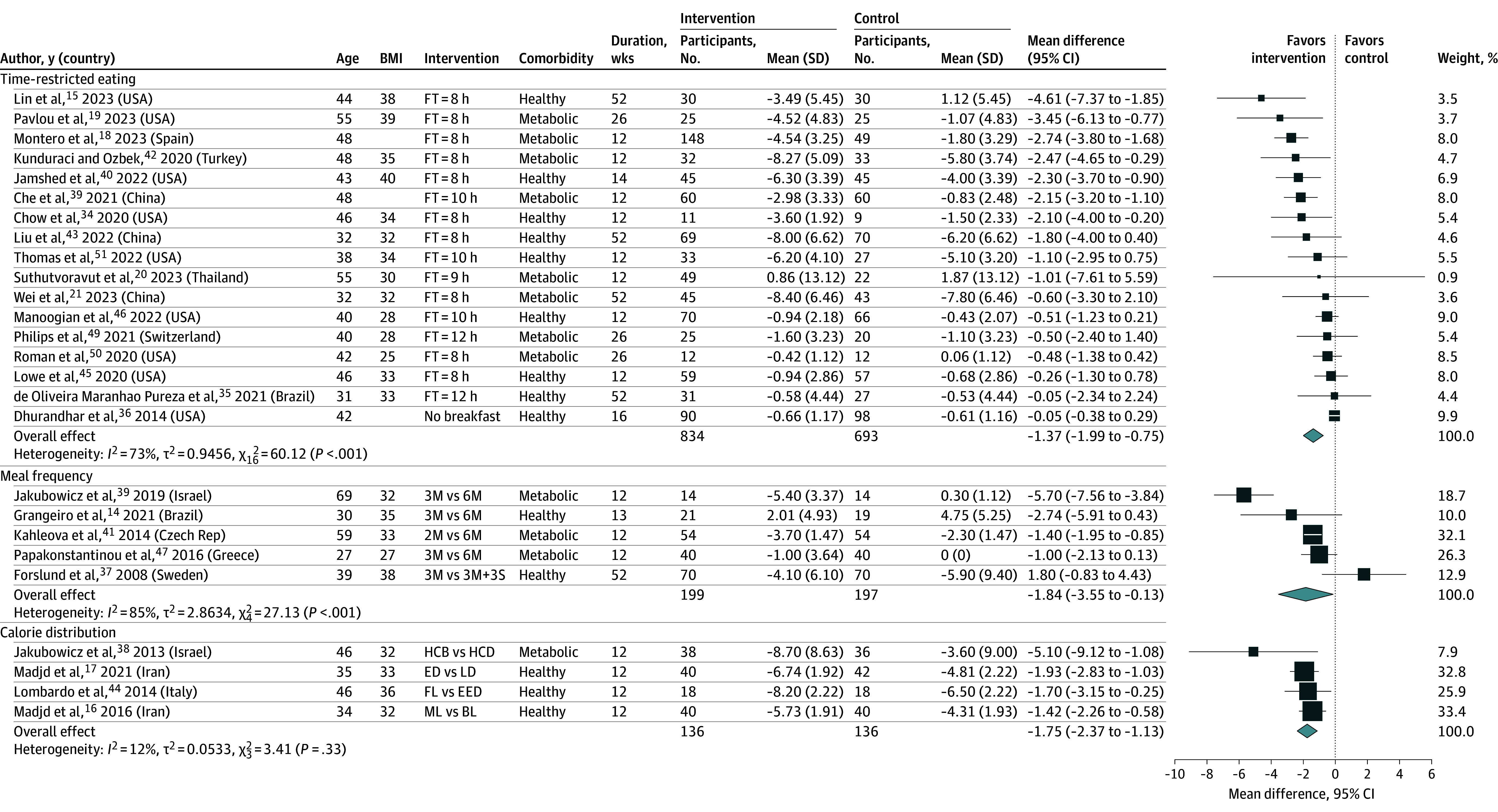
The forest plot shows effect estimates (squares) and 95% CIs (horizontal lines) for each randomized clinical trial (RCT). Larger squares indicate a larger weight has been assigned to that RCT. Left of the 0 line shows a finding in favor of interventions, whereas right of the 0 line shows a finding in favor of control. The diamond at the base of each plot demonstrates the pooled effect estimates and confidence intervals from all RCTs included in the meta-analysis. 2M/3M/6M, 2, 3, or 6 meals; 3M+3S, 3 meals and 3 snacks; BL, back loading (eating the most substantial/calorie-dense meal toward the end of the day); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FT, feeding time; ED, early dinner; EED, equal energy distribution (spreading calorie intake evenly throughout the day’s meals); FL, front loading (consuming the largest or most calorie-dense meal early in the day, typically at breakfast or breakfast and lunch); HCB, high-calorie breakfast; HCD, high-calorie dinner; LD, late dinner; ML, middle loading (eating the most substantial/calorie-dense meal in the middle of the day, usually at lunch).
Table 2. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Summary of Findings and Certainty of Evidence for Meal Timing for Anthropometric and Metabolic Measures.
| Outcomes | Follow-up | No. of participants (studies) | Certainty of the evidence (GRADE)a | Mean difference (95% CI) |
|---|---|---|---|---|
| Time-restricted eating | ||||
| Weight, kg | Follow-up: range 12 to 52 wk | 1527 (17 RCTs) | Lowb,c | –1.37 (–1.99 to –0.75) |
| Relative weight loss, % | Follow-up: range 12 to 52 wk | 770 (8 RCTs) | Lowb,c | −1.82 (−2.81 to −0.83 ) |
| BMId | Follow-up: range 12 to 52 wk | 993 (14 RCTs) | Lowb,c | –0.44 (–0.67 to –0.2) |
| Lean mass, kg | Follow-up: range 12 to 52 wk | 858 (11 RCTs) | Moderateb | –0.42 (–0.7 to –0.15) |
| Waist circumference, cm | Follow-up: range 14 to 52 wk | 530 (7 RCTs) | Lowb | –1.96 (–3.24 to –0.68) |
| HbA1c, % | Follow-up: range 12 to 52 wk | 1022 (13 RCTs) | Lowb,c | –0.08 (–0.15 to –0.01) |
| Fasting plasma glucose, mg/dL | Follow-up: range 12 to 52 wk | 1161 (14 RCTs) | Moderateb | –1.15 (–1.77 to –0.53) |
| LDL, mg/dL | Follow-up: range 12 to 52 wk | 1151 (13 RCTs) | Lowb,d | –1.51 (–1.3 to 4.32) |
| Systolic blood pressure, mmHg | Follow-up: range 12 to 52 wk | 1065 (13 RCTs) | Lowb,c,d | –0.54 (–2.42 to 1.33) |
| Diastolic blood pressure, mmHg | Follow-up: range 12 to 52 wk | 1065 (13 RCTs) | Lowb,c,d | –1.14 (–2.41 to 0.14) |
| Energy intake, kcal/d | Follow-up: range 12 to 52 wk | 843 (10 RCTs) | Lowb,c | –164 (–242.21 to –84.85) |
| Meal frequency, lower frequency vs higher frequency | ||||
| Weight, kg | Follow-up: range 12 to 52 wk | 396 (5 RCTs) | Very lowe,f | –1.84 (–3.55 to –0.13) |
| BMId | Follow-up: range 12 to 26 wk | 369 (5 RCTs) | Very lowe,f | –0.65 (–1.09 to– 0.21) |
| Lean mass, kg | Follow-up: range 13 to 26 wk | 91 (2 RCTs) | Very lowe,f | 1.35 (–0.18 to 2.88) |
| Waist circumference, cm | Follow-up: range 12 to 13 wk | 228 (3 RCTs) | Very lowe,f,g | –0.83 (–4.34 to 2.68) |
| HbA1c, % | Follow-up: range 12 to 12 wk | 310 (4 RCTs) | Very lowe,f,g | –0.14 (–0.39 to 0.11) |
| Fasting glucose, mg/dL | Follow-up: range 12 to 52 wk | 490 (6 RCTs) | Very lowe,f,g | –5.4 (–17.22 to 6.42) |
| LDL, mg/dL | Follow-up: range 12 to 52 wk | 458 (5 RCTs) | Very lowe,f,g | 4.27 (–3.34 to 11.87) |
| Systolic blood pressure, mmHg | Follow-up: mean 52 wk | 140 (1 RCT) | Very lowe,g | 0.7 (–3.28 to 4.68) |
| Diastolic blood pressure, mmHg | Follow-up: mean 52 wk | 140 (1 RCT) | Very lowe,g | –0.1 (–3.45 to 3.25) |
| Energy intake, kcal/d | Follow-up: range 12 to 26 wk | 159 (2 RCTs) | Very lowf,g | –0.64 (–208.34 to 207.07) |
| Calorie distribution, distribution of calories earlier vs later in the biological day | ||||
| Weight, kg | Follow-up: range 12 to 12 wk | 272 (4 RCTs) | Lowe | –1.75 (–2.37 to –1.13) |
| BMId | Follow-up: range 12 to 12 wk | 272 (4 RCTs) | Very lowe,f | –1.06 (–1.82 to –0.3) |
| Waist circumference, cm | Follow-up: range 12 to 12 wk | 272 (4 RCTs) | Very lowc,e | –1.77 (–2.89 to –0.65) |
| HbA1c, % | Follow-up: range 12 to 12 wk | 162 (2 RCTs) | Very lowb,g | –0.01% (–0.06 to 0.04) |
| Fasting glucose, mg/dL | Follow-up: range 12 to 12 wk | 272 (4 RCTs) | Very lowe,f,g | –3.06 (–6.73 to 0.6) |
| LDL, mg/dL | Follow-up: range 12 to 12 wk | 272 (4 RCTs) | Very lowe,f,g | –3.95 (–11.67 to 3.77) |
| Systolic blood pressure, mmHg | Follow-up: range 12 to 12 wk | 110 (2 RCTs) | Very lowe,h | –4.96 (–8.54 to –1.38) |
| Diastolic blood pressure, mmHg | Follow-up: range 12 to 12 wk | 110 (2 RCTs) | Very lowc,e,g | –4.64 (–10.79 to 1.51) |
| Energy intake, kcal/d | Follow-up: range 12 to 12 wk | 80 (1 RCT) | Very lowb,c,g | –51 (–96.6 to –5.4) |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein cholesterol; RCT, randomized clinical trial.
SI conversion factors: To convert fasting glucose to mmol/L, multiply by 0.0555. To convert LDL-cholesterol to mmol/L, multiply by 0.0259.
GRADE Working Group grades of evidence: high certainty: high confidence that the true effect lies close to that of the estimate of the effect; moderate certainty: moderate confidence that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: the true effect may be substantially different from the estimate of the effect; very low certainty: the true effect is likely to be substantially different from the estimate of effect. RCTs were downgraded from an initial high rating if a serious flaw was present in any of the following domains: risk of bias (eg, large proportion of information from studies at high risk of bias that is sufficient to affect the interpretation of results), inconsistency (ie, substantial unexplained heterogeneity I2>75%), indirectness (ie, major limitations of the generalizability of the results), imprecision (ie, 95% CIs overlap with minimally important difference for benefits or harms), and publication bias (or small study effect, where >25% of participants were from small studies with <100 participants).
Risk of bias was assessed as serious due to many trials with concerns primarily related to blinding and missing data; these studies were rated down by 1 level for risk of bias.
Inconsistency was assessed as serious due to dissimilarities in point estimates, lack of overlap in CIs, and statistical evidence of heterogeneity; these studies were rated down by 1 level for inconsistency.
Calculated as weight in kilograms divided by height in meters squared.
Risk of bias was assessed as very serious due to many trials with concerns primarily related to blinding and missing data; these studies were rated down by 2 levels for risk of bias.
Inconsistency was assessed as very serious due to dissimilarities in point estimates, lack of overlap in CIs, and statistical evidence of heterogeneity; these studies were rated down by 2 levels for inconsistency.
Imprecision was assessed as very serious because the 95% CI included a point of no difference and failed to exclude important benefits; these studies were rated down by 2 levels for imprecision.
Imprecision was assessed as serious because the 95% CI included a point of no difference and failed to exclude important benefits; these studies were rated down by 1 level for imprecision.
A meta-analysis of 12 RCTs15,19,21,33,34,35,42,43,45,46,49,50 (851 participants) found an association between TRE and reduced BMI (MD, –0.52; 95% CI, –0.78 to –0.26; I2 = 48%; low-certainty evidence) (eFigure 5 in Supplement 1). Baseline BMI status can partly explain the heterogeneity (P for interaction = .01) (eFigure 6 in Supplement 1).
A meta-analysis of 11 RCTs15,18,19,21,34,40,42,43,45,49,51 (858 participants) that evaluated the association of TRE with lean mass found reduced lean mass (MD, –0.42 kg; 95% CI, –0.69 to –0.10 kg; I2 = 0%; moderate-certainty evidence) (eFigure 7 in Supplement 1). Similarly, a meta-analysis of 7 RCTs15,19,21,35,40,43,49 (530 participants) showed that TRE was associated with reduced waist circumference (MD, –1.96 cm; 95% CI, –3.24 to –0.68 cm; I2 = 42%; low-certainty evidence) (eFigure 8 in Supplement 1). No subgroup differences were observed (eFigures 9-28 in Supplement 1). A meta-analysis of 8 RCTs (770 participants) showed that TRE was associated with reduced percentage of weight loss by an MD of 1.82% (95% CI, –2.81% to –0.83%; I2 = 66%; low-certainty evidence) (eFigure 29 in Supplement 1).
Metabolic Measures
Overall, 13 RCTs15,18,19,20,21,33,34,40,42,45,46,49,51 (1022 participants) and 14 RCTs15,18,19,20,21,33,34,40,42,43,45,46,49,51 (1151 participants) were included in the meta-analysis of the association of TRE with HbA1c and fasting glucose, respectively. TRE was associated with reductions in both HbA1c (MD, –0.08%; 95% CI, –0.15% to –0.01%; I2 = 85%; low-certainty evidence) (Figure 3)15,18,19,20,21,33,34,40,42,45,46,49,51 and plasma glucose (MD, –1.15 mg/dL; 95% CI, –1.77 to −0.53 mg/dL; I2 = 0%; low-certainty evidence) (eFigure 30 in Supplement 1).15,18,19,20,21,33,34,40,42,43,45,46,49,51 The gender of the participants (P for interaction = .03) and the clinicians who delivered the intervention (P for interaction = .03) can partly explain the heterogeneity on the effect of TRE on HbA1c (eFigures 31 and 32 in Supplement 1).
Figure 3. Meta-Analysis of Difference in Mean Difference (95% CIs) for the Effect of Meal Timing Interventions on HbA1c (%), Grouped by the Nature of the Meal Timing Intervention.
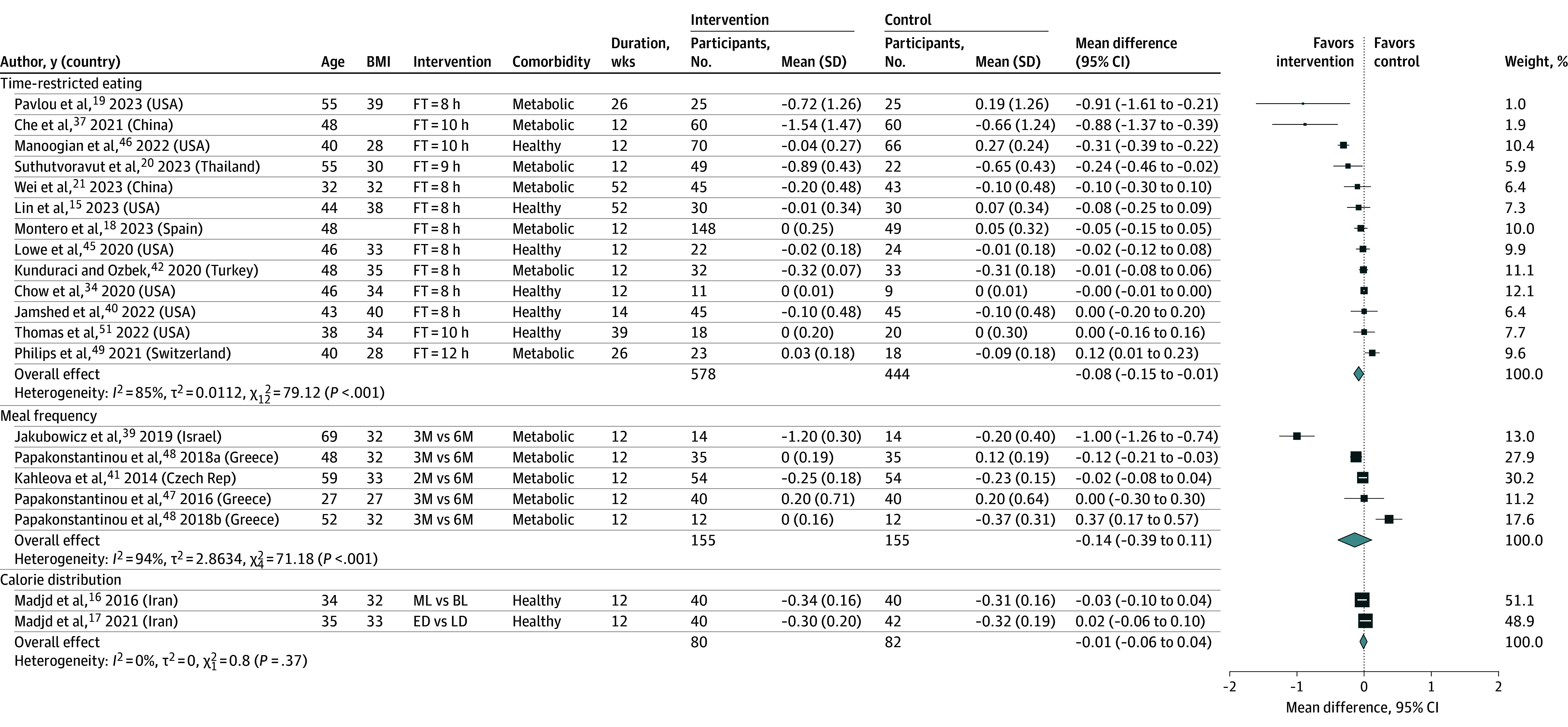
The forest plot shows effect estimates (squares) and 95% CIs (horizontal lines) for each randomized clinical trial (RCT). Larger squares indicate a larger weight has been assigned to that RCT. Left of the 0 line shows a finding in favor of interventions, whereas right of the 0 line shows a finding in favor of control. The diamond at the base of each plot demonstrates the pooled effect estimates and confidence intervals from all RCTs included in the meta-analysis. 2M/3M/6M indicates 2, 3, or 6 meals; BL, back loading (eating the heaviest/most calorie-dense meal toward the end of the day); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ED, early dinner; FT, feeding time; duration, follow-up duration in weeks; LD, late dinner; ML, middle loading (having the most substantial/calorie-dense meal in the middle of the day, usually at lunch).
A meta-analysis of 13 RCTs15,18,19,20,21,33,34,40,42,43,45,46,51 (1151 participants) showed that TRE was associated with reduced LDL (MD, –1.51 mg/dL; 95% CI, –1.30 to 4.32 mg/dL; I2 = 0%; low-certainty evidence) (eFigure 33 in Supplement 1). No subgroup differences were observed (eFigures 34-43 in Supplement 1). Similarly, a meta-analysis of 10 RCTs15,19,20,21,33,40,42,43,46,51 (843 participants) showed that TRE was associated with reduced energy intake (MD, –164 kcal/d; 95% CI, –242 to –85 kcal/d; I2 = 45%; low-certainty evidence) (eFigure 44 in Supplement 1). A subgroup analysis showed that RCTs where participants were allowed to eat freely in both groups resulted in greater reductions in energy intake compared with those with energy-restricted diets (P for interaction = .01) (eFigure 45 in Supplement 1).
Thirteen RCTs15,18,19,20,21,34,35,40,42,43,45,46,49 (1065 participants) reported on the effect of TRE on systolic (SBP) and diastolic blood pressure (DBP). TRE was not associated with change in SBP (MD, –0.54 mm Hg; 95% CI, –2.42 to 1.33 mm Hg; I2 = 38%; low-certainty evidence) (eFigure 46 in Supplement 1) and DBP (MD, –1.14 mm Hg; 95% CI, –2.41 to 0.14 mm Hg; I2 = 22%; low-certainty evidence) (eFigure 47 in Supplement 1). No subgroup differences were observed except for larger reductions in SBP among RCTs that involved clinicians trained specifically in nutrition to deliver the intervention (P for interaction = .02) (eFigures 48-67 and eTables 5-8 in Supplement 1).
Meal Frequency
Anthropometric Measures
Five RCTs were included in the meta-analyses of meal frequency on weight change14,37,39,41,47 and BMI.14,32,41,47,52 Lower meal frequency was associated with small reductions in weight (MD, –1.84 kg; 95% CI, –3.55 to –0.13 kg; I2 = 85%; low-certainty evidence) (Figure 2)14,37,39,41,47 and BMI (MD, 0.65; 95% CI, –1.09 to –0.21; I2 = 76%; low-certainty evidence) (eFigure 3 in Supplement 1).14,32,41,47,52 In subgroup analysis, we found that comorbidity (P for interaction < .001) and intervention intensity (P for interaction = .01) can partly explain the heterogeneity on the effect of meal frequency on BMI (eFigures 68 and 69 in Supplement 1).
We included 2 RCTs14,32 (91 participants) and 3 RCTs14,41,47 (228 participants) that reported on the effect of meal frequency on lean mass and waist circumference, respectively. No clear association was found between meal frequency and lean mass (MD, 1.35 kg; 95% CI, –0.18 to 2.88 kg; I2 = 0%; very low-certainty evidence) (eFigure 7 in Supplement 1)14,32 and waist circumference (MD, –0.83 cm; 95% CI, –4.34 to 2.68 cm; I2 = 97%; very low-certainty evidence)14,41,47 (eFigure 8 in Supplement 1).
Metabolic Measures
No clear association was found between meal frequency and HbA1c (MD, −0.14%; 95% CI, –0.39 to 0.11; I2 = 94%; 310 participants; 4 RCTs; very low-certainty evidence) (Figure 3),39,41,47,48 fasting glucose (MD, –5.4 mg/dL; 95% CI, –17.2 to 6.4 mg/dL; I2 = 100%; 490 participants; 6 RCTs; very low-certainty evidence) (eFigure 30 in Supplement 1),14,37,39,41,47,48 LDL (MD, 4.27 mg/dL; 95% CI, –3.34 to 11.87 mg/dL; I2 = 82%; 458 participants; 5 RCTs; very low-certainty evidence) (eFigure 33 in Supplement 1),14,37,41,47,52 SBP (MD, 0.7 mm Hg; 95% CI, –3.28 to 4.68 mm Hg; I2 = NA; 140 participants; 1 RCT; very low-certainty evidence) (eFigure 46 in Supplement 1),37 DBP (MD, –0.1 mm Hg; 95% CI, –3.45 to 3.25 mm Hg; I2 = NA; 140 participants; 1 RCT; very low-certainty evidence) (eFigure 47 in Supplement 1),37 and energy intake (MD, –0.64 kcal/d; 95% CI, –208.3 to 207.1 kcal/d; I2 = 0%; 159 participants; 2 RCTs; very low-certainty evidence) (eFigure 44 in Supplement 1).32,41
Calorie Distribution
Anthropometric Measures
A meta-analysis of 4 RCTs16,17,38,44 (272 participants) that evaluated the association of calorie distribution across the biological day with weight showed that consuming the majority of calories earlier in the day resulted in more weight loss compared with consuming them later in the day (MD, –1.75 kg; 95% CI, –2.37 to –1.13 kg; I2 = 12%; low-certainty evidence) (Figure 2). Calorie distribution (eg, consuming most calorie-dense meal[s] earlier vs later in the biological day, also known as front-loading vs back-loading calories) was associated with reduced BMI (MD, –1.06; 95% CI, –1.82 to –0.30; I2 = 91%; 272 participants; 4 RCTs; very low-certainty evidence) (eFigure 3 in Supplement 1)16,17,38,44 and waist circumference (MD, –1.77 cm; 95% CI, –2.89 to –0.65; I2 = 53%; 272 participants; 4 RCTs; very low-certainty evidence) (eFigure 8 in Supplement 1)16,17,38,44; however, the evidence is very uncertain.
Metabolic Measures
No clear association was found between calorie distribution and HbA1c (MD, –0.01%; 95% CI, –0.06 to 0.04; I2 = 0%; 162 participants; 2 RCTs; very low-certainty evidence) (eFigure 30 in Supplement 1),16,17 fasting glucose (MD, –3.06 mg/dL; 95% CI, –6.73 to 0.60 mg/dL; I2 = 95%; 272 participants; 4 RCTs; very low-certainty evidence) (eFigure 30 in Supplement 1),16,17,38,44 LDL (MD, –3.95 mg/dL; 95% CI, –11.67 to 3.77 mg/dL; I2 = 95%; 272 participants; 4 RCTs; very low-certainty evidence) (eFigure 33 in Supplement 1),16,17,38,44 SBP (MD, –4.96 mm Hg; 95% CI, –8.54 to –1.38 mm Hg; I2 = 22%; 110 participants; 2 RCTs; very low-certainty evidence) (eFigure 46 in Supplement 1),38,44 DBP (MD, –4.64 mm Hg; 95% CI, –10.79 to 1.51 mm Hg; I2 = 51%; 110 participants; 2 RCTs; very low-certainty evidence) (eFigure 47 in Supplement 1),38,44 and energy intake (MD, –51 kcal/d; 95% CI, –97 to –5 kcal/d; I2 = NA; 80 participants; 1 RCT; very low-certainty evidence) (eFigure 44 in Supplement 1).16 We could not draw robust conclusions regarding the association of calorie distribution with metabolic measures (eFigures 70-185 in Supplement 1).
Discussion
In this meta-analysis study of RCTs, meal timing strategies were associated with small reductions in body weight, BMI, and waist circumference over more than 12 weeks (low-certainty evidence). Furthermore, our findings suggest that TRE might improve diabetes indicators such as HbA1c and fasting glucose (low-certainty evidence).
Previous systematic reviews of RCTs in adults with obesity found similar results for the effect of TRE and meal frequency on weight loss, but not calorie distribution.4,5,6,7,8,9 Meal frequency and calorie distribution may enhance weight loss by aligning with circadian rhythms, improving metabolic efficiency, regulating appetite hormones, and reducing late-day snacking behaviors.53
We also found TRE-mediated effects on metabolic indicators were mostly consistent with previous systematic reviews whereby TRE significantly reduced fasting glucose but not LDL or BP, although none of the previous reviews reported improvement in HbA1c.7,8,11,12 A recent review6 found no significant impact of meal frequency on glycemic control, but it did observe an effect on LDL levels. A systematic review54 estimated that for each kilogram of weight loss, HbA1c reduced by a mean of 0.1%. Collectively, our findings provide new insights on the longer-term effects of these interventions on weight loss and/or management.
The rigid nature of calorie counting in traditional weight loss interventions is often associated with higher disinhibition, energy intake, and BMI.55 Therefore, regular dietetic counselling is considered important for sustained weight management.56 However, results from our subgroup analysis showed that the benefits of meal timing on weight loss outcomes were not altered by how trained clinicians were or frequency of support provided. Although weight loss was not clinically significant (<5%),57 TRE may provide a simpler and more flexible approach for health care clinicians to support behavioral change in adults with BMI 25 to 40.
Limitations
Our review has several limitations. First, most studies recruited participants from clinical settings (only 2 from community43,46) and involved clinicians with nutrition training, which might limit generalizability. Additionally, all studies on calorie distribution involved female participants only, which limited the generalizability to male populations.
Second, the overall quality of evidence was low because of the risk of bias and inconsistency. Most included studies rated as high risk14,16,17,18,20,32,33,34,36,37,38,39,41,42,44,46,47,48,49,50,51,52 because of the difficulty in blinding dietary interventions and the use of self-reported outcome measures. Our results were limited by the high heterogeneity, which was partly explained by subgroup analyses. TRE was also the dominant intervention, with limited studies on meal frequency and calorie distribution. Finally, some studies did not adequately report outcome data. However, we extracted necessary data from figures or requested it from authors. Further trials without prescribed energy restriction, larger sample sizes, similar intervention designs, and longer follow-up periods are needed.
Conclusions
This meta-analysis found that TRE, lower meal frequency, or consuming calories earlier in the day was associated with a small amount of weight loss and improved metabolic function. Although effect sizes were small, these strategies may be plausible for sustained weight reduction.
eTable 1. Search strategy and key concepts/MeSH
eTable 2. Notable studies excluded with reasoning
eTable 3. Included studies
eTable 4. Details of Intervention Delivery
eTable 5. Adherence outcomes
eTable 6. Hunger outcomes
eTable 7. Satiety outcomes
eTable 8. Notable clinical trials excluded
eFigure 1. Risk of summary for included studies
eFigure 2. Risk of bias graph for included studies
eFigure 3. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on weight (kg), grouped by baseline BMI status
eFigure 4. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating interventions on weight (kg), grouped by eating window
eFigure 5. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on BMI (kg/m2), grouped by intervention type
eFigure 6. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on BMI (kg/m2), grouped by baseline BMI status
eFigure 7. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on lean mass (kg), grouped by the nature of the meal timing intervention
eFigure 8. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on waist circumference (cm), grouped by meal timing intervention
eFigure 9. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by gender proportion
eFigure 10. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by baseline BMI status
eFigure 11. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by energy prescription
eFigure 12. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by follow duration
eFigure 13. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by health status
eFigure 14. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by eating window
eFigure 15. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by delivery personnel
eFigure 16. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by frequency of contact
eFigure 17. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by resource provision
eFigure 18. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by risk of bias
eFigure 19. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by gender proportion
eFigure 20. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by baseline BMI status
eFigure 21. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by health status
eFigure 22. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by energy prescription
eFigure 23. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by follow duration
eFigure 24. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by eating window
eFigure 25. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by frequency of contact
eFigure 26. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by delivery personnel
eFigure 27. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by resource provision
eFigure 28. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by risk of bias
eFigure 29. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on HbA1c (%), grouped by the meal timing intervention
eFigure 30. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on fasting plasma glucose (mg/dL), grouped by the meal timing intervention
eFigure 31. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by gender proportion
eFigure 32. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by delivery personnel
eFigure 33. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on LDL (mg/dL), grouped by the meal timing intervention
eFigure 34. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by gender proportion
eFigure 35. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by baseline BMI status
eFigure 36. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by health status
eFigure 37. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by energy prescription
eFigure 38. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by follow duration
eFigure 39. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by eating window
eFigure 40. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by frequency of contact
eFigure 41. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by delivery personnel
eFigure 42. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by resource provision
eFigure 43. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by risk of bias
eFigure 44. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on energy intake (kcal/day), grouped by the meal timing intervention
eFigure 45. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by energy prescription
eFigure 46. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on systolic blood pressure (mmHg), grouped by the meal timing intervention
eFigure 47. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on diastolic blood pressure (mmHg), grouped by the meal timing intervention
eFigure 48. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by gender proportion
eFigure 49. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by baseline BMI status
eFigure 50. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by health status
eFigure 51. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by energy prescription
eFigure 52. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by follow duration
eFigure 53. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by eating window
eFigure 54. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by frequency of contact
eFigure 55. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by delivery personnel
eFigure 56. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by resource provision
eFigure 57. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by risk of bias
eFigure 58. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by gender proportion
eFigure 59. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by baseline BMI status
eFigure 60. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by health status
eFigure 61. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by energy prescription
eFigure 62. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by follow duration
eFigure 63. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by eating window
eFigure 64. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by frequency of contact
eFigure 65. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by delivery personnel
eFigure 66. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by resource provision
eFigure 67. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by risk of bias
eFigure 68. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by health status
eFigure 69. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by intervention intensity
eFigure 70. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by gender proportion
eFigure 71. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by health status
eFigure 72. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by energy prescription
eFigure 73. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by follow duration
eFigure 74. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by frequency of contact
eFigure 75. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by delivery personnel
eFigure 76. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by resource provision
eFigure 77. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by risk of bias
eFigure 78. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by gender proportion
eFigure 79. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by baseline BMI status
eFigure 80. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by health status
eFigure 81. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by follow duration
eFigure 82. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by intervention intensity
eFigure 83. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by frequency of contact
eFigure 84. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by delivery personnel
eFigure 85. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by baseline BMI status
eFigure 86. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by health status
eFigure 87. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by delivery personnel
eFigure 88. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by resource provision
eFigure 89. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by gender proportion
eFigure 90. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by health status
eFigure 91. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by energy prescription
eFigure 92. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by follow duration
eFigure 93. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by eating window
eFigure 94. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by frequency of contact
eFigure 95. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by delivery personnel
eFigure 96. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by resource provision
eFigure 97. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by risk of bias
eFigure 98. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by gender proportion
eFigure 99. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by baseline BMI status
eFigure 100. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by follow duration
eFigure 101. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by frequency of contact
eFigure 102. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by delivery personnel
eFigure 103. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by resource provision
eFigure 104. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by baseline BMI status
eFigure 105. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by health status
eFigure 106. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by delivery personnel
eFigure 107. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by resource provision
eFigure 108. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by gender proportion
eFigure 109. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by follow duration
eFigure 110. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by intervention intensity
eFigure 111. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by frequency of contact
eFigure 112. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by resource provision
eFigure 113. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by gender proportion
eFigure 114. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by baseline BMI status
eFigure 115. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by health status
eFigure 116. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by intervention intensity
eFigure 117. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by delivery personnel
eFigure 118. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by baseline BMI status
eFigure 119. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by health status
eFigure 120. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by delivery personnel
eFigure 121. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by resource provision
eFigure 122. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by gender proportion
eFigure 123. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by baseline BMI status
eFigure 124. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by health status
eFigure 125. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by follow duration
eFigure 126. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by eating window
eFigure 127. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by frequency of contact
eFigure 128. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by delivery personnel
eFigure 129. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by resource provision
eFigure 130. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by risk of bias
eFigure 131. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by baseline BMI status
eFigure 132. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by health status
eFigure 133. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by energy prescription
eFigure 134. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by follow duration
eFigure 135. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by eating window
eFigure 136. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by frequency of contact
eFigure 137. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by resource provision
eFigure 138. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by risk of bias
eFigure 139. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by gender proportion
eFigure 140. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by baseline BMI status
eFigure 141. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by intervention intensity
eFigure 142. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by frequency of contact
eFigure 143. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by delivery personnel
eFigure 144. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by gender proportion
eFigure 145. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 146. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by health status
eFigure 147. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by energy prescription
eFigure 148. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by follow duration
eFigure 149. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by eating window
eFigure 150. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by frequency of contact
eFigure 151. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 152. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by resource provision
eFigure 153. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by risk of bias
eFigure 154. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by gender proportion
eFigure 155. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 156. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by health status
eFigure 157. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by follow duration
eFigure 158. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by intervention intensity
eFigure 159. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by frequency of contact
eFigure 160. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 161. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 162. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by health status
eFigure 163. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 164. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by resource provision
eFigure 165. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by gender proportion
eFigure 166. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by baseline BMI status
eFigure 167. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by health status
eFigure 168. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by follow duration
eFigure 169. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by intervention intensity
eFigure 170. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by frequency of contact
eFigure 171. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by delivery personnel
eFigure 172. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by resource provision
eFigure 173. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by baseline BMI status
eFigure 174. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by health status
eFigure 175. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by delivery personnel
eFigure 176. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by resource provision
eFigure 177. Funnel plots for weight (TRE vs control)
eFigure 178. Funnel plots for BMI (TRE vs control)
eFigure 179. Funnel plots for lean mass (TRE vs control)
eFigure 180. Funnel plots for energy intake (TRE vs control)
eFigure 181. Funnel plots for HbA1c (TRE vs control)
eFigure 182. Funnel plots for fasting glucose (TRE vs control)
eFigure 183. Funnel plots for LDL (TRE vs control)
eFigure 184. Funnel plots for systolic blood pressure (TRE vs control)
eFigure 185. Funnel plots for diastolic blood pressure (TRE vs control)
Data Sharing Statement
References
- 1.World Health Organisation . Obesity and overweight. Updated March 1, 2024. Accessed March 14, 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al. ; Global BMI Mortality Collaboration . Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-786. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao AM, Quigley KM, Wadden TA. Dietary interventions for obesity: clinical and mechanistic findings. J Clin Invest. 2021;131(1):e140065. doi: 10.1172/JCI140065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hareer LW, Lau YY, Mole F, et al. The effectiveness of the Mediterranean diet for primary and secondary prevention of cardiovascular disease: an umbrella review. Nutr Diet. Published online August 14, 2024. doi: 10.1111/1747-0080.12891 [DOI] [PubMed] [Google Scholar]
- 5.Greenwood H, Barnes K, Clark J, Ball L, Albarqouni L. Long-term effect of salt substitution for cardiovascular outcomes: a systematic review and meta-analysis. Ann Intern Med. 2024;177(5):643-655. doi: 10.7326/M23-2626 [DOI] [PubMed] [Google Scholar]
- 6.Abdollahi S, Kazemi A, de Souza RJ, Clark CCT, Soltani S. The effect of meal frequency on biochemical cardiometabolic factors: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2021;40(5):3170-3181. doi: 10.1016/j.clnu.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 7.Chen JH, Lu LW, Ge Q, et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2023;63(15):2331-2347. doi: 10.1080/10408398.2021.1974335 [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Liu X, Bao L, Yang P, Zhou H. Health effects of the time-restricted eating in adults with obesity: a systematic review and meta-analysis. Front Nutr. 2023;10:1079250. doi: 10.3389/fnut.2023.1079250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong M, Caterson ID, Madigan CD. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br J Nutr. 2017;118(8):616-628. doi: 10.1017/S0007114517002550 [DOI] [PubMed] [Google Scholar]
- 10.Schwingshackl L, Nitschke K, Zähringer J, et al. Impact of meal frequency on anthropometric outcomes: a systematic review and network meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1108-1122. doi: 10.1093/advances/nmaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JC, Tan ZT, He CJ, Hu HL, Zhai CL, Qian G. Time-restricted eating with calorie restriction on weight loss and cardiometabolic risk: a systematic review and meta-analysis. Eur J Clin Nutr. 2023;77(11):1014-1025. doi: 10.1038/s41430-023-01311-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamarul Zaman M, Teng NIMF, Kasim SS, Juliana N, Alshawsh MA. Effects of time-restricted eating with different eating duration on anthropometrics and cardiometabolic health: a systematic review and meta-analysis. World J Cardiol. 2023;15(7):354-374. doi: 10.4330/wjc.v15.i7.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. doi: 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grangeiro ÉD, Trigueiro MS, Siais LO, et al. Hypocaloric diet with lower meal frequency did not affect weight loss, body composition and insulin responsiveness, but improved lipid profile: a randomized clinical trial. Food Funct. 2021;12(24):12594-12605. doi: 10.1039/D1FO00484K [DOI] [PubMed] [Google Scholar]
- 15.Lin S, Cienfuegos S, Ezpeleta M, et al. Time-restricted eating without calorie counting for weight loss in a racially diverse population: a randomized controlled trial. Ann Intern Med. 2023;176(7):885-895. doi: 10.7326/M23-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weight-loss program: a randomized clinical trial. Am J Clin Nutr. 2016;104(4):982-989. doi: 10.3945/ajcn.116.134163 [DOI] [PubMed] [Google Scholar]
- 17.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects of consuming later evening meal v. earlier evening meal on weight loss during a weight loss diet: a randomised clinical trial. Br J Nutr. 2021;126(4):632-640. doi: 10.1017/S0007114520004456 [DOI] [PubMed] [Google Scholar]
- 18.Montero MD. Effects of three 8-hour time-restricted eating schedules on visceral adipose tissue, body composition and cardiometabolic health in men and women with overweight/obesity: a multicenter randomized controlled trial. University of Granada . 2023. Accessed September 25, 2024. https://digibug.ugr.es/bitstream/handle/10481/84704/89159.pdf?sequence=4andisAllowed=y
- 19.Pavlou V, Cienfuegos S, Lin S, et al. Effect of time-restricted eating on weight loss in adults with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. 2023;6(10):e2339337. doi: 10.1001/jamanetworkopen.2023.39337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suthutvoravut U, Anothaisintawee T, Boonmanunt S, et al. Efficacy of time-restricted eating and behavioral economic intervention in reducing fasting plasma glucose, HbA1c, and cardiometabolic risk factors in patients with impaired fasting glucose: a randomized controlled trial. Nutrients. 2023;15(19):4233. doi: 10.3390/nu15194233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Lin B, Huang Y, et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD randomized clinical trial. JAMA Netw Open. 2023;6(3):e233513. doi: 10.1001/jamanetworkopen.2023.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane Handbook for Systematic Reviews of Interventions version 6.4. 2023. Accessed October 2, 2024. https://training.cochrane.org/handbook
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81-90. doi: 10.1016/j.jclinepi.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Li T, Deeks JJ, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 64. Cochrane; 2023. [Google Scholar]
- 26.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Savovoć J, Page MJ, Elbers RG, Sterne JA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 64. Cochrane; 2023. [Google Scholar]
- 28.Schünemann HJ, Higgins JP, Vist GE, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 64. Cochrane; 2023. [Google Scholar]
- 29.Santesso N, Glenton C, Dahm P, et al. ; GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126-135. doi: 10.1016/j.jclinepi.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 30.Manager R. 5 (RevMan 5). The Cochrane Collaboration; 2020. [Google Scholar]
- 31.Fischer L, Frank P. AMA Manual of Style: A Guide for Authors and Editors. Oxford University Press; 2020. [Google Scholar]
- 32.Bachman JL, Raynor HA. Effects of manipulating eating frequency during a behavioral weight loss intervention: a pilot randomized controlled trial. Obesity (Silver Spring). 2012;20(5):985-992. doi: 10.1038/oby.2011.360 [DOI] [PubMed] [Google Scholar]
- 33.Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond). 2021;18(1):88. doi: 10.1186/s12986-021-00613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow LS, Manoogian ENC, Alvear A, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860-869. doi: 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, et al. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: a 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759-766. doi: 10.1016/j.clnu.2020.06.036 [DOI] [PubMed] [Google Scholar]
- 36.Dhurandhar EJ, Dawson J, Alcorn A, et al. The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100(2):507-513. doi: 10.3945/ajcn.114.089573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertéus Forslund H, Klingström S, Hagberg H, Löndahl M, Torgerson JS, Lindroos AK. Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur J Clin Nutr. 2008;62(11):1308-1317. doi: 10.1038/sj.ejcn.1602860 [DOI] [PubMed] [Google Scholar]
- 38.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504-2512. doi: 10.1002/oby.20460 [DOI] [PubMed] [Google Scholar]
- 39.Jakubowicz D, Landau Z, Tsameret S, et al. Reduction in glycated hemoglobin and daily insulin dose alongside circadian clock upregulation in patients with type 2 diabetes consuming a three-meal diet: a randomized clinical trial. Diabetes Care. 2019;42(12):2171-2180. doi: 10.2337/dc19-1142 [DOI] [PubMed] [Google Scholar]
- 40.Jamshed H, Steger FL, Bryan DR, et al. Effectiveness of early time-restricted eating for weight loss, fat loss, and cardiometabolic health in adults with obesity: a randomized clinical trial. JAMA Intern Med. 2022;182(9):953-962. doi: 10.1001/jamainternmed.2022.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahleova H, Belinova L, Malinska H, et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57(8):1552-1560. doi: 10.1007/s00125-014-3253-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunduraci YE, Ozbek H. Does the energy restriction intermittent fasting diet alleviate metabolic syndrome biomarkers? a randomized controlled trial. Nutrients. 2020;12(10):3213. doi: 10.3390/nu12103213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495-1504. doi: 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 44.Lombardo M, Bellia A, Padua E, et al. Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr. 2014;33(3):198-205. doi: 10.1080/07315724.2013.863169 [DOI] [PubMed] [Google Scholar]
- 45.Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491-1499. doi: 10.1001/jamainternmed.2020.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoogian ENC, Zadourian A, Lo HC, et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022;34(10):1442-1456.e7. doi: 10.1016/j.cmet.2022.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papakonstantinou E, Kechribari I, Mitrou P, et al. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial. Eur J Clin Nutr. 2016;70(5):588-594. doi: 10.1038/ejcn.2015.225 [DOI] [PubMed] [Google Scholar]
- 48.Papakonstantinou E, Kontogianni MD, Mitrou P, et al. Effects of 6 vs 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 diabetes: a randomized trial. Diabetes Metab. 2018;44(3):226-234. doi: 10.1016/j.diabet.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 49.Phillips NE, Mareschal J, Schwab N, et al. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients. 2021;13(3):1042. doi: 10.3390/nu13031042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roman SN, Fitzgerald KC, Beier M, Mowry EM. Safety and feasibility of various fasting-mimicking diets among people with multiple sclerosis. Mult Scler Relat Disord. 2020;42:102149. doi: 10.1016/j.msard.2020.102149 [DOI] [PubMed] [Google Scholar]
- 51.Thomas EA, Zaman A, Sloggett KJ, et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity (Silver Spring). 2022;30(5):1027-1038. doi: 10.1002/oby.23420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatami Zargaran Z, Salehi M, Heydari ST, Babajafari S. The effects of 6 isocaloric meals on body weight, lipid profiles, leptin, and adiponectin in overweight subjects (BMI > 25). Int Cardiovasc Res J. 2014;8(2):52-56. [PMC free article] [PubMed] [Google Scholar]
- 53.Davis R, Rogers M, Coates AM, Leung GKW, Bonham MP. The impact of meal timing on risk of weight gain and development of obesity: a review of the current evidence and opportunities for dietary intervention. Curr Diab Rep. 2022;22(4):147-155. doi: 10.1007/s11892-022-01457-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gummesson A, Nyman E, Knutsson M, Karpefors M. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1295-1305. doi: 10.1111/dom.12971 [DOI] [PubMed] [Google Scholar]
- 55.Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. Int J Eat Disord. 1999;26(1):53-64. doi: [DOI] [PubMed] [Google Scholar]
- 56.Machado AM, Guimarães NS, Bocardi VB, et al. Understanding weight regain after a nutritional weight loss intervention: systematic review and meta-analysis. Clin Nutr ESPEN. 2022;49:138-153. doi: 10.1016/j.clnesp.2022.03.020 [DOI] [PubMed] [Google Scholar]
- 57.Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi: 10.1080/00325481.2022.2051366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search strategy and key concepts/MeSH
eTable 2. Notable studies excluded with reasoning
eTable 3. Included studies
eTable 4. Details of Intervention Delivery
eTable 5. Adherence outcomes
eTable 6. Hunger outcomes
eTable 7. Satiety outcomes
eTable 8. Notable clinical trials excluded
eFigure 1. Risk of summary for included studies
eFigure 2. Risk of bias graph for included studies
eFigure 3. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on weight (kg), grouped by baseline BMI status
eFigure 4. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating interventions on weight (kg), grouped by eating window
eFigure 5. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on BMI (kg/m2), grouped by intervention type
eFigure 6. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on BMI (kg/m2), grouped by baseline BMI status
eFigure 7. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on lean mass (kg), grouped by the nature of the meal timing intervention
eFigure 8. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on waist circumference (cm), grouped by meal timing intervention
eFigure 9. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by gender proportion
eFigure 10. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by baseline BMI status
eFigure 11. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by energy prescription
eFigure 12. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by follow duration
eFigure 13. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by health status
eFigure 14. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by eating window
eFigure 15. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by delivery personnel
eFigure 16. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by frequency of contact
eFigure 17. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by resource provision
eFigure 18. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on lean mass (kg), grouped by risk of bias
eFigure 19. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by gender proportion
eFigure 20. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by baseline BMI status
eFigure 21. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by health status
eFigure 22. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by energy prescription
eFigure 23. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by follow duration
eFigure 24. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by eating window
eFigure 25. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by frequency of contact
eFigure 26. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by delivery personnel
eFigure 27. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by resource provision
eFigure 28. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on waist circumference (cm), grouped by risk of bias
eFigure 29. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on HbA1c (%), grouped by the meal timing intervention
eFigure 30. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on fasting plasma glucose (mg/dL), grouped by the meal timing intervention
eFigure 31. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by gender proportion
eFigure 32. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by delivery personnel
eFigure 33. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on LDL (mg/dL), grouped by the meal timing intervention
eFigure 34. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by gender proportion
eFigure 35. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by baseline BMI status
eFigure 36. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by health status
eFigure 37. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by energy prescription
eFigure 38. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by follow duration
eFigure 39. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by eating window
eFigure 40. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by frequency of contact
eFigure 41. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by delivery personnel
eFigure 42. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by resource provision
eFigure 43. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on LDL (mg/dL), grouped by risk of bias
eFigure 44. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on energy intake (kcal/day), grouped by the meal timing intervention
eFigure 45. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by energy prescription
eFigure 46. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on systolic blood pressure (mmHg), grouped by the meal timing intervention
eFigure 47. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal timing interventions on diastolic blood pressure (mmHg), grouped by the meal timing intervention
eFigure 48. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by gender proportion
eFigure 49. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by baseline BMI status
eFigure 50. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by health status
eFigure 51. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by energy prescription
eFigure 52. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by follow duration
eFigure 53. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by eating window
eFigure 54. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by frequency of contact
eFigure 55. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by delivery personnel
eFigure 56. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by resource provision
eFigure 57. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on systolic blood pressure (mmHg), grouped by risk of bias
eFigure 58. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by gender proportion
eFigure 59. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by baseline BMI status
eFigure 60. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on diastolic blood pressure (mmHg), grouped by health status
eFigure 61. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by energy prescription
eFigure 62. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by follow duration
eFigure 63. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by eating window
eFigure 64. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by frequency of contact
eFigure 65. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by delivery personnel
eFigure 66. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by resource provision
eFigure 67. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on Diastolic blood pressure (mmHg), grouped by risk of bias
eFigure 68. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by health status
eFigure 69. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by intervention intensity
eFigure 70. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by gender proportion
eFigure 71. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by health status
eFigure 72. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by energy prescription
eFigure 73. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by follow duration
eFigure 74. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by frequency of contact
eFigure 75. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by delivery personnel
eFigure 76. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by resource provision
eFigure 77. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on weight (kg), grouped by risk of bias
eFigure 78. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by gender proportion
eFigure 79. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by baseline BMI status
eFigure 80. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by health status
eFigure 81. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by follow duration
eFigure 82. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by intervention intensity
eFigure 83. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by frequency of contact
eFigure 84. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on weight (kg), grouped by delivery personnel
eFigure 85. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by baseline BMI status
eFigure 86. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by health status
eFigure 87. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by delivery personnel
eFigure 88. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on weight (kg), grouped by resource provision
eFigure 89. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by gender proportion
eFigure 90. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by health status
eFigure 91. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by energy prescription
eFigure 92. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by follow duration
eFigure 93. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by eating window
eFigure 94. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by frequency of contact
eFigure 95. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by delivery personnel
eFigure 96. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by resource provision
eFigure 97. Meta-analysis of difference in mean difference (95% CIs) for the effect of time restricted eating on BMI (kg/m2), grouped by risk of bias
eFigure 98. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by gender proportion
eFigure 99. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by baseline BMI status
eFigure 100. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by follow duration
eFigure 101. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by frequency of contact
eFigure 102. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by delivery personnel
eFigure 103. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on BMI (kg/m2), grouped by resource provision
eFigure 104. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by baseline BMI status
eFigure 105. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by health status
eFigure 106. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by delivery personnel
eFigure 107. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on BMI (kg/m2), grouped by resource provision
eFigure 108. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by gender proportion
eFigure 109. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by follow duration
eFigure 110. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by intervention intensity
eFigure 111. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by frequency of contact
eFigure 112. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on lean mass (kg), grouped by resource provision
eFigure 113. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by gender proportion
eFigure 114. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by baseline BMI status
eFigure 115. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by health status
eFigure 116. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by intervention intensity
eFigure 117. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on waist circumference (cm), grouped by delivery personnel
eFigure 118. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by baseline BMI status
eFigure 119. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by health status
eFigure 120. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by delivery personnel
eFigure 121. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on waist circumference (cm), grouped by resource provision
eFigure 122. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by gender proportion
eFigure 123. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by baseline BMI status
eFigure 124. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by health status
eFigure 125. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by follow duration
eFigure 126. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by eating window
eFigure 127. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by frequency of contact
eFigure 128. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by delivery personnel
eFigure 129. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by resource provision
eFigure 130. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on energy intake (kcal/day), grouped by risk of bias
eFigure 131. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by baseline BMI status
eFigure 132. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by health status
eFigure 133. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by energy prescription
eFigure 134. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by follow duration
eFigure 135. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by eating window
eFigure 136. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by frequency of contact
eFigure 137. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by resource provision
eFigure 138. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on HbA1c (%), grouped by risk of bias
eFigure 139. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by gender proportion
eFigure 140. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by baseline BMI status
eFigure 141. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by intervention intensity
eFigure 142. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by frequency of contact
eFigure 143. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on HbA1c (%), grouped by delivery personnel
eFigure 144. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by gender proportion
eFigure 145. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 146. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by health status
eFigure 147. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by energy prescription
eFigure 148. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by follow duration
eFigure 149. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by eating window
eFigure 150. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by frequency of contact
eFigure 151. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 152. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by resource provision
eFigure 153. Meta-analysis of difference in mean difference (95% CIs) for the effect of time-restricted eating on fasting glucose (mg/dL), grouped by risk of bias
eFigure 154. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by gender proportion
eFigure 155. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 156. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by health status
eFigure 157. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by follow duration
eFigure 158. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by intervention intensity
eFigure 159. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by frequency of contact
eFigure 160. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 161. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by baseline BMI status
eFigure 162. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by health status
eFigure 163. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by delivery personnel
eFigure 164. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on fasting glucose (mg/dL), grouped by resource provision
eFigure 165. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by gender proportion
eFigure 166. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by baseline BMI status
eFigure 167. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by health status
eFigure 168. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by follow duration
eFigure 169. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by intervention intensity
eFigure 170. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by frequency of contact
eFigure 171. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by delivery personnel
eFigure 172. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal frequency on LDL (mg/dL), grouped by resource provision
eFigure 173. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by baseline BMI status
eFigure 174. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by health status
eFigure 175. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by delivery personnel
eFigure 176. Meta-analysis of difference in mean difference (95% CIs) for the effect of meal distribution on LDL (mg/dL), grouped by resource provision
eFigure 177. Funnel plots for weight (TRE vs control)
eFigure 178. Funnel plots for BMI (TRE vs control)
eFigure 179. Funnel plots for lean mass (TRE vs control)
eFigure 180. Funnel plots for energy intake (TRE vs control)
eFigure 181. Funnel plots for HbA1c (TRE vs control)
eFigure 182. Funnel plots for fasting glucose (TRE vs control)
eFigure 183. Funnel plots for LDL (TRE vs control)
eFigure 184. Funnel plots for systolic blood pressure (TRE vs control)
eFigure 185. Funnel plots for diastolic blood pressure (TRE vs control)
Data Sharing Statement