Abstract
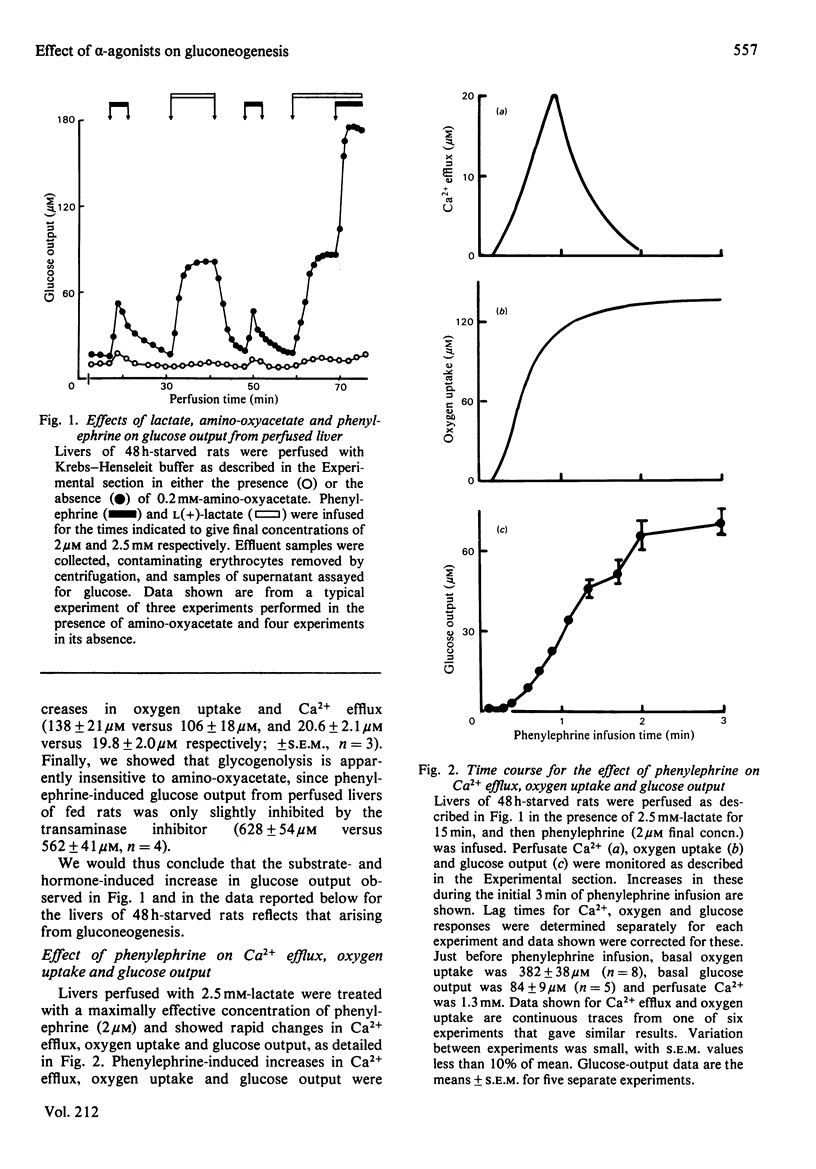
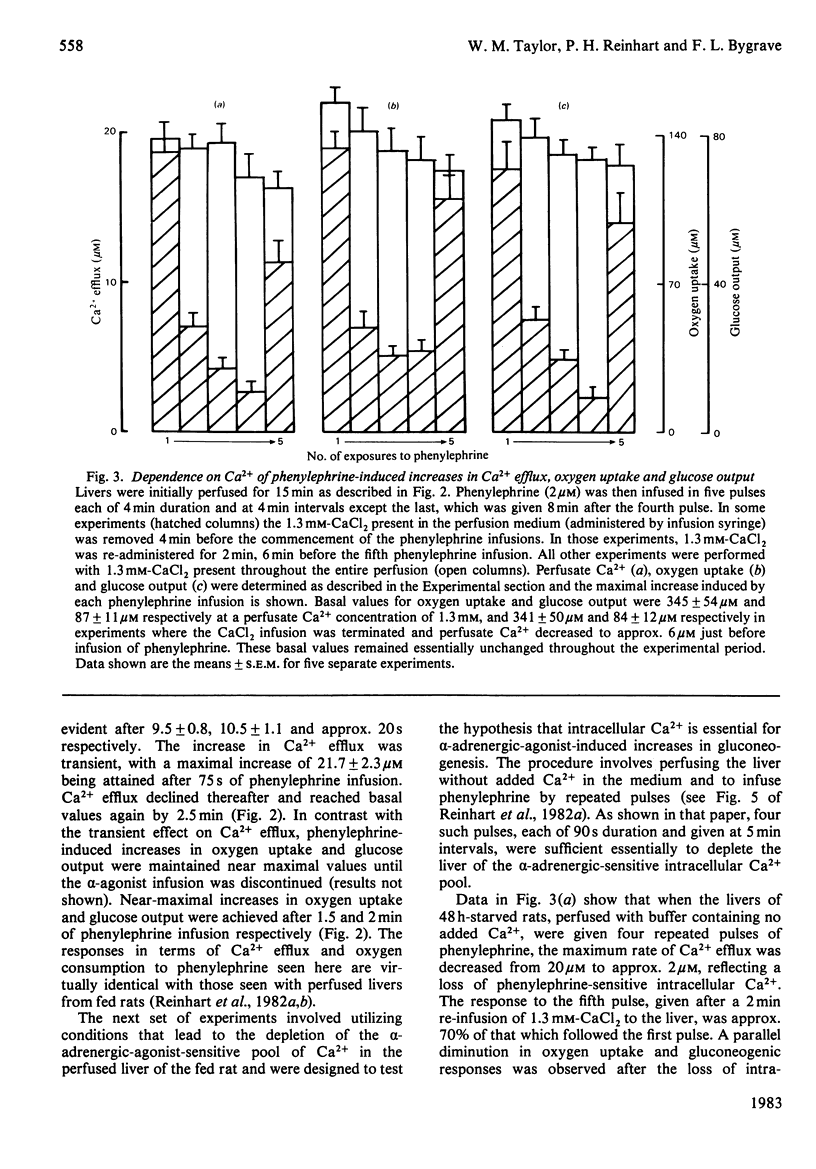
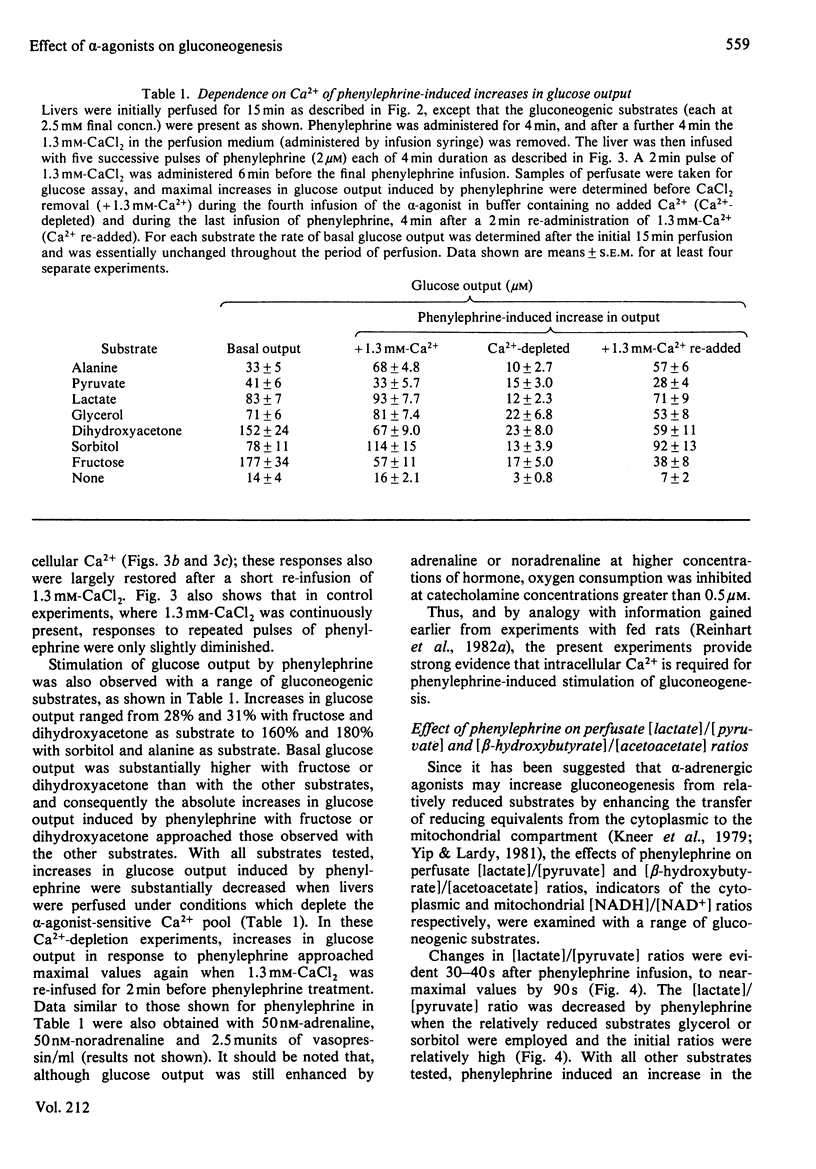
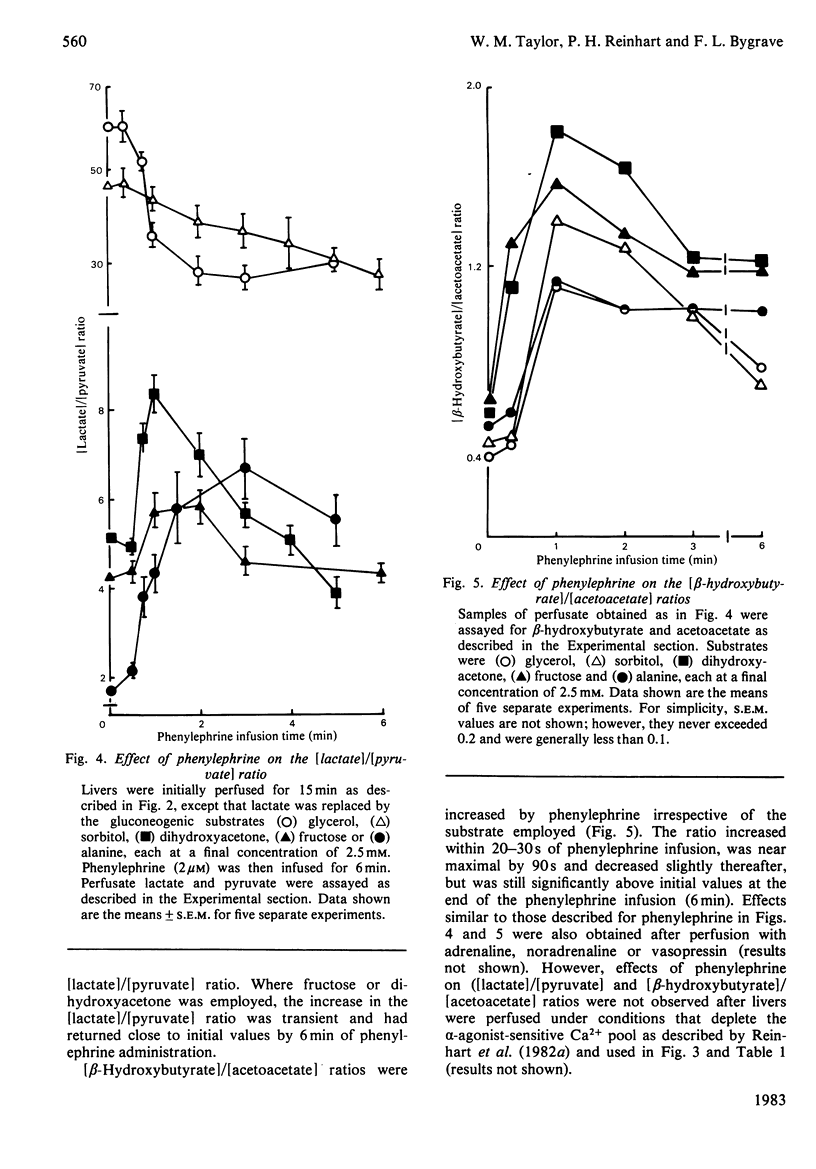
Glucose output from perfused livers of 48 h-starved rats was stimulated by phenylephrine (2 microM) when lactate, pyruvate, alanine, glycerol, sorbitol, dihydroxyacetone or fructose were used as gluconeogenic precursors. Phenylephrine-induced increases in glucose output were immediately preceded by a transient efflux of Ca2+ and a sustained increase in oxygen uptake. Phenylephrine decreased the perfusate [lactate]/[pyruvate] ratio when sorbitol or glycerol was present, but increased the ratio when alanine, dihydroxyacetone or fructose was present. Phenylephrine induced a rapid increase in the perfusate [beta-hydroxybutyrate]/[acetoacetate] ratio and increased total ketone-body output by 40-50% with all substrates. The oxidation of [1-14C]octanoate or 2-oxo[1-14C]glutarate to 14CO2 was increased by up to 200% by phenylephrine. All responses to phenylephrine infusion were diminished after depletion of the hepatic alpha-agonist-sensitive pool of Ca2+ and returned toward maximal responses after Ca2+ re-addition. Phenylephrine-induced increases in glucose output from lactate, sorbitol and glycerol were inhibited by the transaminase inhibitor amino-oxyacetate by 95%, 75% and 66% respectively. Data presented suggest that the mobilization of an intracellular pool of Ca2+ is involved in the activation of gluconeogenesis by alpha-adrenergic agonists in perfused rat liver. alpha-Adrenergic activation of gluconeogenesis is apparently accompanied by increases in fatty acid oxidation and tricarboxylic acid-cycle flux. An enhanced transfer of reducing equivalents from the cytoplasmic to the mitochondrial compartment may also be involved in the stimulation of glucose output from the relatively reduced substrates glycerol and sorbitol and may arise principally from an increased flux through the malate-aspartate shuttle.
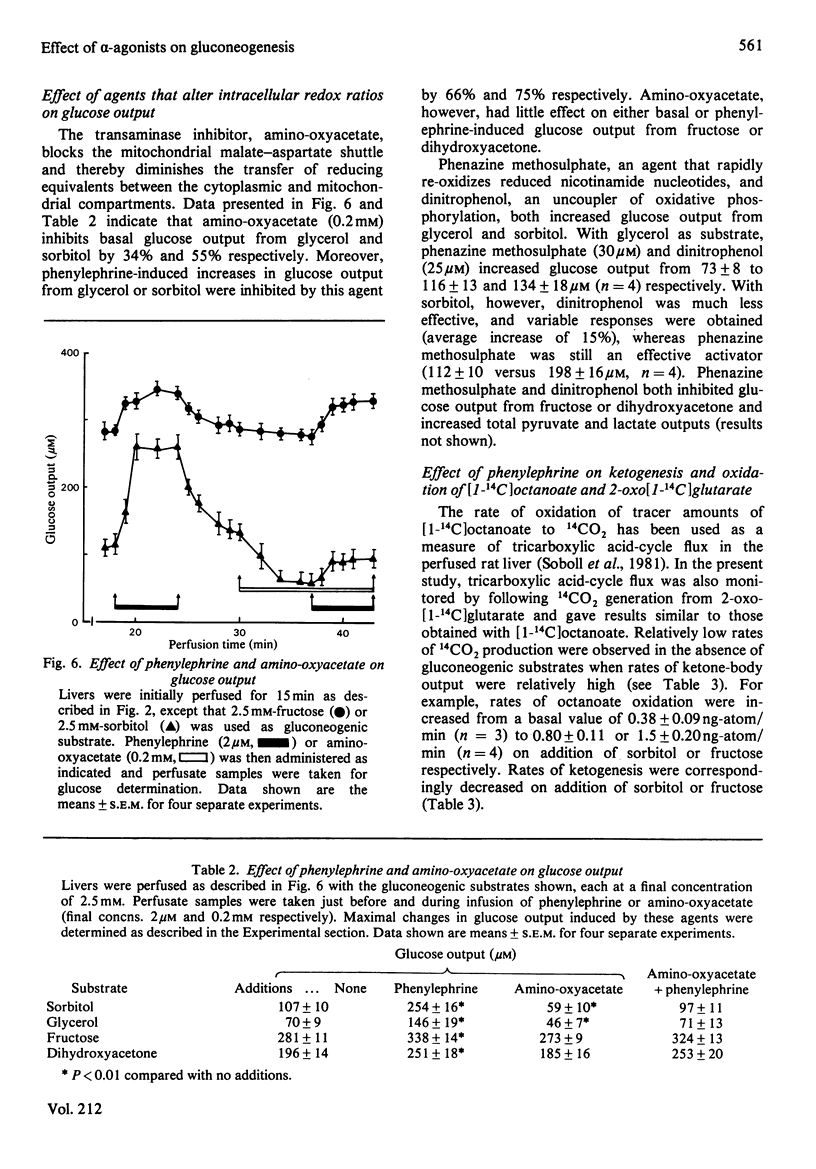
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arinze I. J., Garber A. J., Hanson R. W. The regulation of gluconeogenesis in mammalian liver. The role of mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1973 Apr 10;248(7):2266–2274. [PubMed] [Google Scholar]
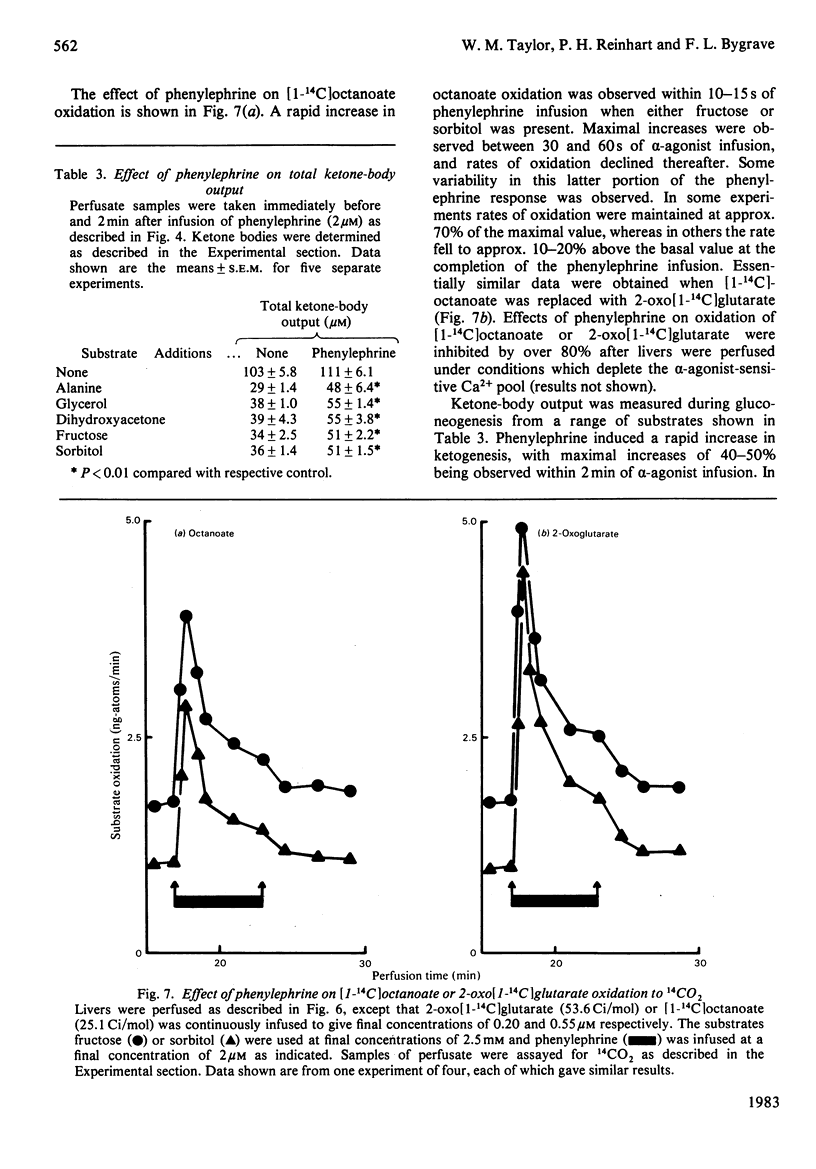
- Cederbaum A. I., Dicker E., Rubin E. Transfer and reoxidation of reducing equivalents as the rate-limiting steps in the oxidation of ethanol by liver cells isolated from fed and fasted rats. Arch Biochem Biophys. 1977 Oct;183(2):638–646. doi: 10.1016/0003-9861(77)90398-8. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Friedmann N., Rasmussen H. Calcium, manganese and hepatic gluconeogenesis. Biochim Biophys Acta. 1970 Oct 27;222(1):41–52. doi: 10.1016/0304-4165(70)90349-1. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K., Florio V. A., Twible D. A. The role of calcium ion as a mediator of the effects of angiotensin II, catecholamines, and vasopressin on the phosphorylation and activity of enzymes in isolated hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7147–7156. [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K. Regulation of mitochondrial pyruvate carboxylation and gluconeogenesis in rat hepatocytes via an alpha-adrenergic, adenosine 3':5'-monophosphate-independent mechanism. J Biol Chem. 1979 Feb 25;254(4):1129–1133. [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneer N. M., Wagner M. J., Lardy H. A. Regulation by calcium of hormonal effects on gluconeogenesis. J Biol Chem. 1979 Dec 10;254(23):12160–12168. [PubMed] [Google Scholar]
- Ly S., Kim K. H. Inactivation of hepatic acetyl-CoA carboxylase by catecholamine and its agonists through the alpha-adrenergic receptors. J Biol Chem. 1981 Nov 25;256(22):11585–11590. [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. A comparative study of the regulation of Ca2+ of the activities of the 2-oxoglutarate dehydrogenase complex and NAD+-isocitrate dehydrogenase from a variety of sources. Biochem J. 1981 May 15;196(2):619–624. doi: 10.1042/bj1960619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryfield M. L., Lardy H. A. Ca2+-mediated activation of phosphoenolpyruvate carboxykinase occurs via release of Fe2+ from rat liver mitochondria. J Biol Chem. 1982 Apr 10;257(7):3628–3635. [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982 Feb 25;257(4):1906–1912. [PubMed] [Google Scholar]
- Richards C. S., Uyeda K. Hormonal regulation of fructose-6-P-2-kinase and fructose-2,6-P2 by two mechanisms. J Biol Chem. 1982 Aug 10;257(15):8854–8861. [PubMed] [Google Scholar]
- Rognstad R. Effects of ethyl hydrazinoacetate on gluconeogenesis and on ethanol oxidation in rat hepatocytes. Biochim Biophys Acta. 1980 Feb 21;628(1):116–118. doi: 10.1016/0304-4165(80)90357-8. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Comparative studies on the influence of hormones on metabolite compartmentation in isolated liver cells during gluconeogenesis from lactate. Biochem Soc Trans. 1978;6(6):1139–1144. doi: 10.1042/bst0061139. [DOI] [PubMed] [Google Scholar]
- Soboll S., Heldt H. W., Scholz R. Changes in the subcellular distribution of metabolites due to ethanol oxidation in the perfused rat liver. Hoppe Seylers Z Physiol Chem. 1981 Mar;362(3):247–260. doi: 10.1515/bchm2.1981.362.1.247. [DOI] [PubMed] [Google Scholar]
- Sugano T., Shiota M., Tanaka T., Miyamae Y., Shimada M., Oshino N. Intracellular redox state and stimulation of gluconeogenesis by glucagon and norepinephrine in the perfused rat liver. J Biochem. 1980 Jan;87(1):153–166. doi: 10.1093/oxfordjournals.jbchem.a132721. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Tordoff A. F., Ilic V., Williamson D. H. Alpha-adrenergic stimulation of [1-14C]oleate oxidation to 14CO2 in isolated rat hepatocytes. FEBS Lett. 1980 Oct 20;120(1):80–84. doi: 10.1016/0014-5793(80)81051-9. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Fain J. N. Studies on the regulation of gluconeogenesis in isolated rat liver cells by epinephrine and glucagon. J Biol Chem. 1974 Feb 25;249(4):1162–1166. [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Study of the fructose 6-phosphate/fructose 1,6-bi-phosphate cycle in the liver in vivo. Biochem J. 1980 Oct 15;192(1):263–271. doi: 10.1042/bj1920263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Jakob A., Refino C. Control of the removal of reducing equivalents from the cytosol in perfused rat liver. J Biol Chem. 1971 Dec 25;246(24):7632–7641. [PubMed] [Google Scholar]
- Yip B. P., Lardy H. A. The role of calcium in the stimulation of gluconeogenesis by catecholamines. Arch Biochem Biophys. 1981 Dec;212(2):370–377. doi: 10.1016/0003-9861(81)90377-5. [DOI] [PubMed] [Google Scholar]


