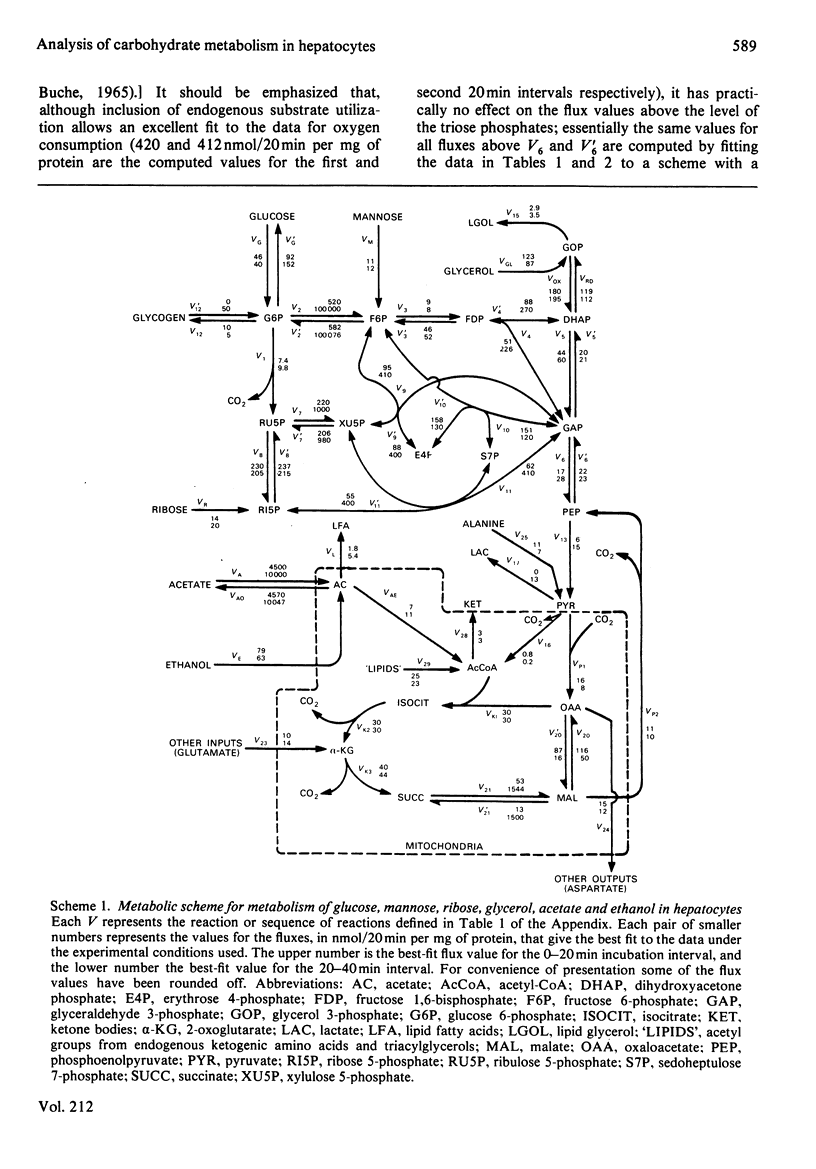
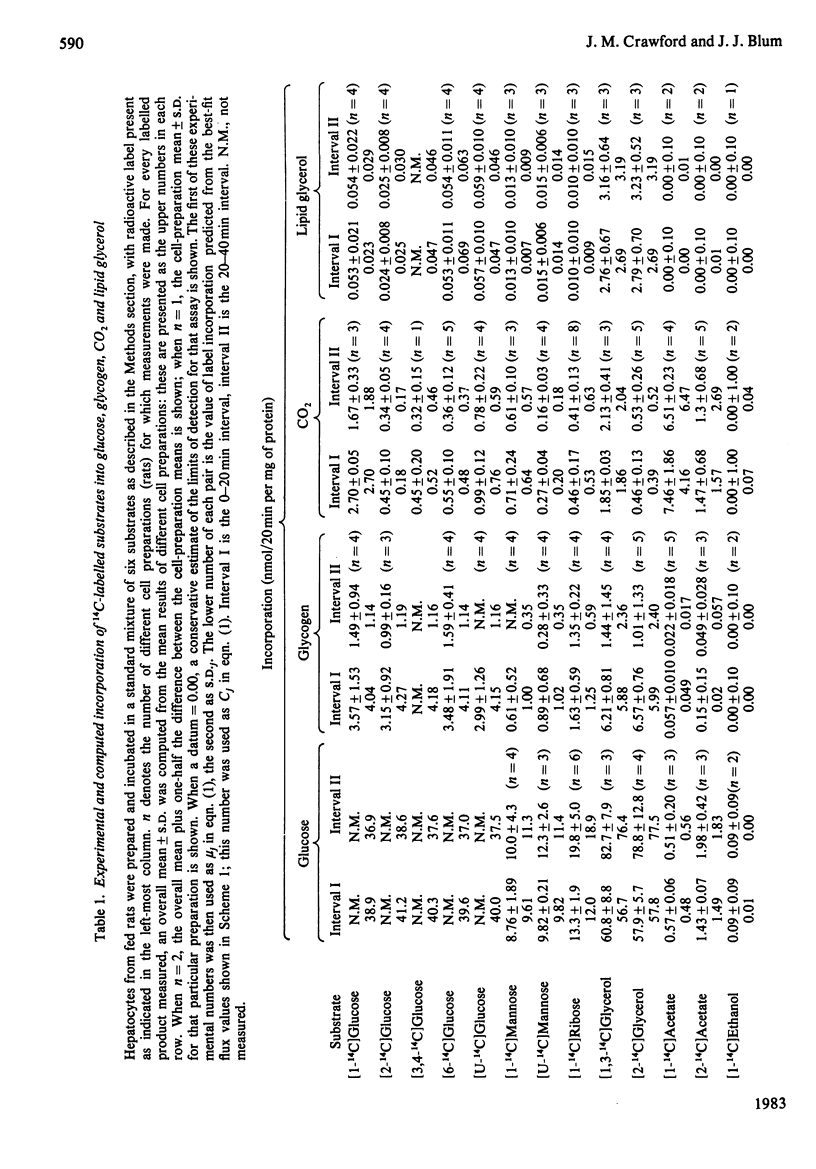
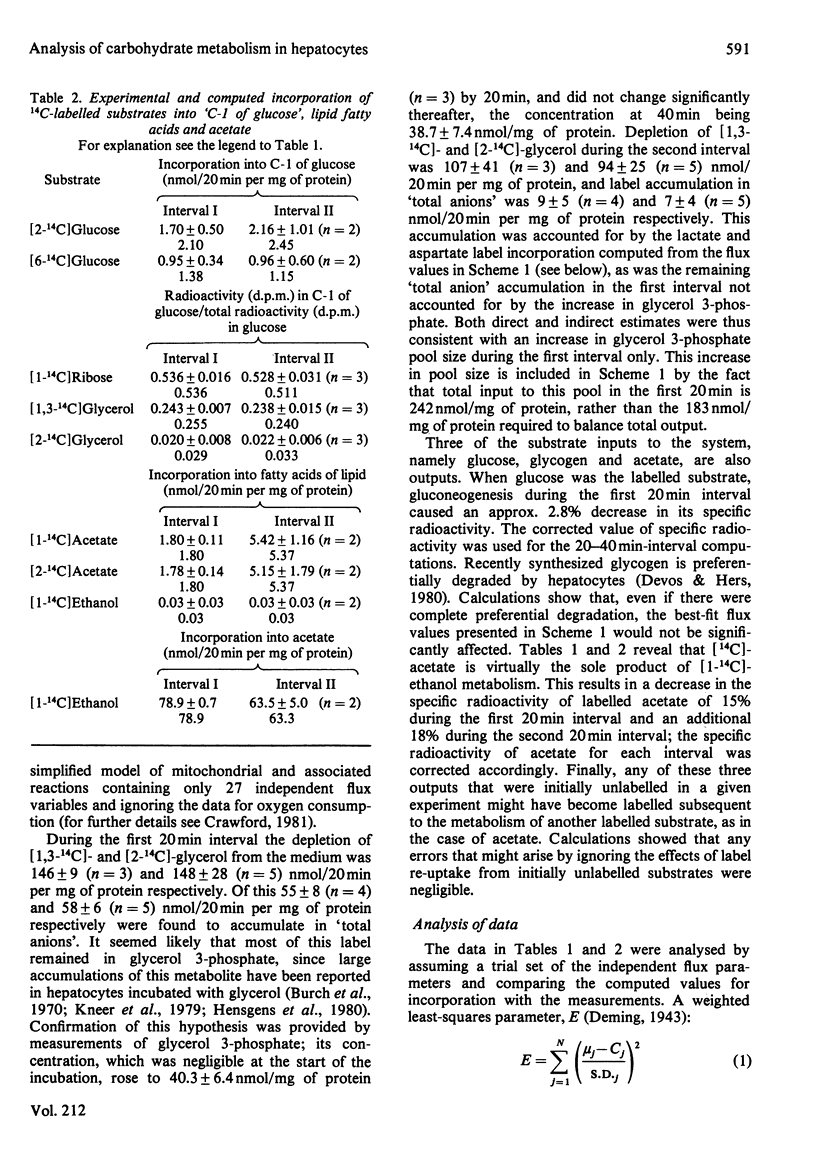
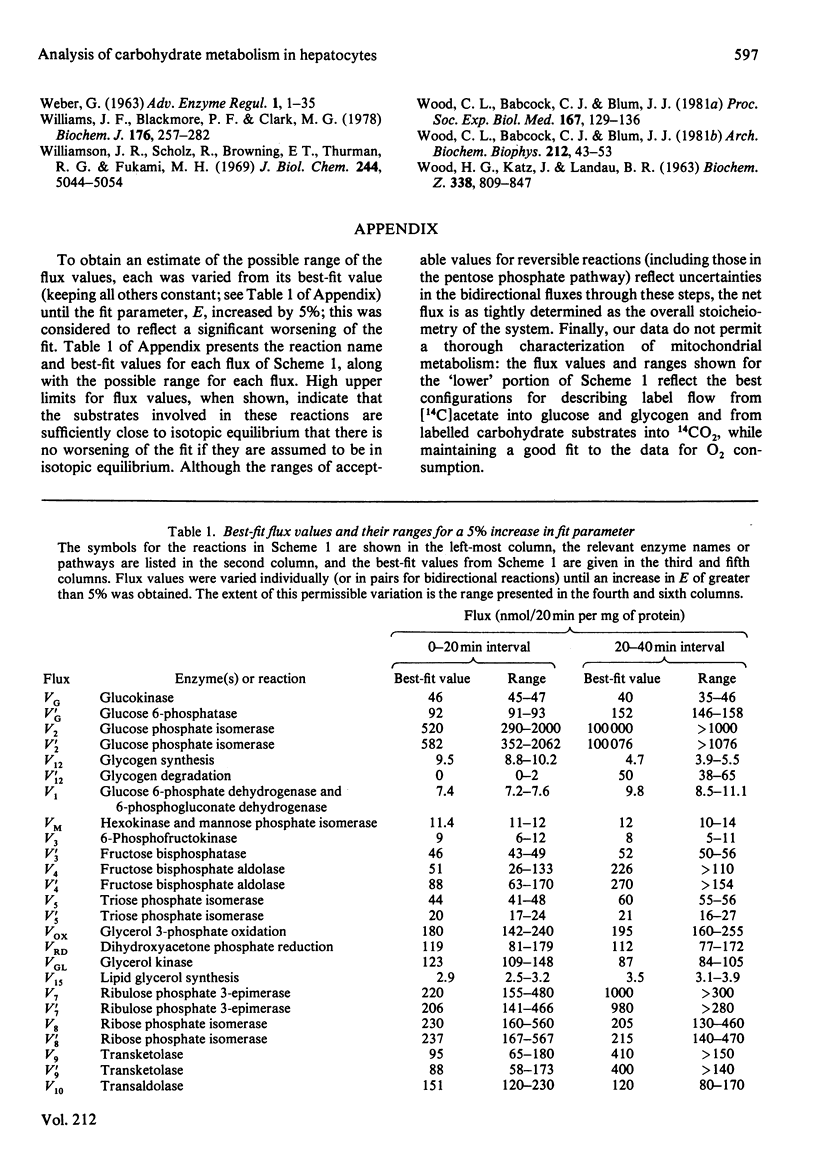
Abstract
Hepatocytes were isolated from the livers of fed rats and incubated with a mixture of glucose (10 mM), ribose (1 mM), mannose (4 mM), glycerol (3 mM), acetate (1.25 mM), and ethanol (5 mM) with one substrate labelled with 14C in any given incubation. Incorporation of label into CO2, glucose, glycogen, lipid glycerol and fatty acids, acetate and C-1 of glucose was measured at 20 and 40 min after the start of the incubation. The data (about 48 measurements for each interval) were used in conjunction with a single-compartment model of the reactions of the gluconeogenic, glycolytic and pentose phosphate pathways and a simplified model of the relevant mitochondrial reactions. An improved method of computer analysis of the equations describing the flow of label through each carbon atom of each metabolite under steady-state conditions was used to compute values for the 34 independent flux parameters in this model. A good fit to the data was obtained, thereby permitting good estimates of most of the fluxes in the pathways under consideration. The data show that: net flux above the level of the triose phosphates is gluconeogenic; label in the hexose phosphates is fully equilibrated by the second 20 min interval; the triose phosphate isomerase step does not equilibrate label between the triose phosphates; substrate cycles are operating at the glucose-glucose 6-phosphate, fructose 6-phosphate-fructose 1,6-bisphosphate and phosphoenolpyruvate-pyruvate-oxaloacetate cycles; and, although net flux through the enzymes catalysing the non-oxidative steps of the pentose phosphate pathway is small, bidirectional fluxes are large.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbritti G., De Matteis F. Decreased levels of cytochrome P-450 and catalase in hepatic porphyria caused by substituted acetamides and barbiturates. Importance of the allyl group in the molecule of the active drugs. Chem Biol Interact. 1972 Mar;4(4):281–286. doi: 10.1016/0009-2797(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Althoff J., Grandjean C., Russell L., Pour P. Vinylethylnitrosamine: a potent respiratory carcinogen in Syrian hamsters. J Natl Cancer Inst. 1977 Feb;58(2):439–442. doi: 10.1093/jnci/58.2.439. [DOI] [PubMed] [Google Scholar]
- Arion W. J., Lange A. J., Walls H. E., Ballas L. M. Evidence for the participation of independent translocation for phosphate and glucose 6-phosphate in the microsomal glucose-6-phosphatase system. Interactions of the system with orthophosphate, inorganic pyrophosphate, and carbamyl phosphate. J Biol Chem. 1980 Nov 10;255(21):10396–10406. [PubMed] [Google Scholar]
- Arion W. J., Wallin B. K., Carlson P. W., Lange A. J. The specificity of glucose 6-phosphatase of intact liver microsomes. J Biol Chem. 1972 Apr 25;247(8):2558–2565. [PubMed] [Google Scholar]
- Augusto O., Beilan H. S., Ortiz de Montellano P. R. The catalytic mechanism of cytochrome P-450. Spin-trapping evidence for one-electron substrate oxidation. J Biol Chem. 1982 Oct 10;257(19):11288–11295. [PubMed] [Google Scholar]
- Baquer N. Z., Cascales M., McLean P., Greenbaum A. L. Effects of thyroid hormone deficiency on the distribution of hepatic metabolites and control of pathways of carbohydrate metabolism in liver and adipose tissue of the rat. Eur J Biochem. 1976 Sep 15;68(2):403–413. doi: 10.1111/j.1432-1033.1976.tb10827.x. [DOI] [PubMed] [Google Scholar]
- Baquer N. Z., McLean P. Evidence for the existence and functional activity of the pentose phosphate pathway in the large particle fraction isolated from rat tissues. Biochem Biophys Res Commun. 1972 Jan 14;46(1):167–174. doi: 10.1016/0006-291x(72)90646-8. [DOI] [PubMed] [Google Scholar]
- Baquer N. Z., Sochor M., McLean P. Hormonal control of the 'compartmentation' of the enzymes of the pentose phosphate pathway associated with the large particle fraction of rat liver. Biochem Biophys Res Commun. 1972 Apr 14;47(1):218–226. doi: 10.1016/s0006-291x(72)80031-7. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Kun E., Werner H. V. Regulatory role of reducing-equivalent transfer from substrate to oxygen in the hepatic metabolism of glycerol and sorbitol. Eur J Biochem. 1973 Mar 15;33(3):407–417. doi: 10.1111/j.1432-1033.1973.tb02697.x. [DOI] [PubMed] [Google Scholar]
- Blattmann L., Joswig N., Preussmann R. Struktur von Metaboliten des carcinogenen Methyl-n-butyl-nitrosamins im Rattenurin. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974 Mar 14;81(1):71–73. doi: 10.1007/BF00303602. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Hue L., Hers H. G. Phosphorylation of glucose in isolated rat hepatocytes. Sigmoidal kinetics explained by the activity of glucokinase alone. Biochem J. 1978 Aug 15;174(2):603–611. doi: 10.1042/bj1740603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz M. J., Stein R. B., Blum J. J. Quantitative analysis of the change of metabolite fluxes along the pentose phosphate and glycolytic pathways in Tetrahymena in response to carbohydrates. J Biol Chem. 1977 Mar 10;252(5):1589–1605. [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clark D. G., Storer G. B., Topping D. L. Inhibition of the substrate cycle glucose:glucose 6-phosphate by physiological concentrations of fructose in perfused rat liver. Biochem Biophys Res Commun. 1980 Mar 13;93(1):155–161. doi: 10.1016/s0006-291x(80)80259-2. [DOI] [PubMed] [Google Scholar]
- Clark D., Lee D., Rognstad R., Katz J. Futile cycles in isolated perfused rat liver and in isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1975 Nov 3;67(1):212–219. doi: 10.1016/0006-291x(75)90304-6. [DOI] [PubMed] [Google Scholar]
- Coffman B. L., Ingall G., Tephly T. R. The formation of N-alkylprotoporphyrin IX and destruction of cytochrome P-450 in the liver of rats after treatment with 3,5-diethoxycarbonyl-1,4-dihydrocollidine and its 4-ethyl analog. Arch Biochem Biophys. 1982 Oct 1;218(1):220–224. doi: 10.1016/0003-9861(82)90339-3. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Blum J. J. On the use of trace levels of [1-14 C] galactose to estimate cycling between fructose 6-phosphate and fructose diphosphate. Arch Biochem Biophys. 1982 Jun;216(1):42–50. doi: 10.1016/0003-9861(82)90186-2. [DOI] [PubMed] [Google Scholar]
- DUTTON A. H., HEATH D. F. Demethylation of dimethylnitrosamine in rats and mice. Nature. 1956 Sep 22;178(4534):644–644. doi: 10.1038/178644a0. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Cantoni L. Alteration of the porphyrin nucleus of cytochrome P-450 caused in the liver by treatment with allyl-containing drugs. Is the modified porphyrin N-substituted? Biochem J. 1979 Oct 1;183(1):99–103. doi: 10.1042/bj1830099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Drug-induced conversion of liver haem into modified porphyrins. Evidence for two classes of products. Biochem J. 1980 Apr 1;187(1):285–288. doi: 10.1042/bj1870285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Farmer P. B., Lamb J. H. Liver production of N-alkylated porphyrins caused in mice by treatment with substituted dihydropyridines. Evidence that the alkyl group on the pyrrole nitrogen atom originates from the drug. FEBS Lett. 1981 Jul 6;129(2):328–331. doi: 10.1016/0014-5793(81)80194-9. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Jackson A. H., Gibbs A. H., Rao K. R., Atton J., Weerasinghe S., Hollands C. Structural isomerism and chirality of N-monosubstituted protoporphyrins. FEBS Lett. 1982 Jun 1;142(1):44–48. doi: 10.1016/0014-5793(82)80216-0. [DOI] [PubMed] [Google Scholar]
- Devos P., Hers H. G. Glycogen in rat adipose tissue: sequential synthesis and random degradation. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1031–1036. doi: 10.1016/0006-291x(80)91576-4. [DOI] [PubMed] [Google Scholar]
- Dyson J. E., Noltmann E. A. The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. Participation of histidine and lysine in a proposed dual function mechanism. J Biol Chem. 1968 Apr 10;243(7):1401–1414. [PubMed] [Google Scholar]
- El-Refai M., Bergman R. N. Simulation study of control of hepatic glycogen synthesis by glucose and insulin. Am J Physiol. 1976 Nov;231(5 Pt 1):1608–1619. doi: 10.1152/ajplegacy.1976.231.5.1608. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Haliday E., Negishi M., Hjelmeland L. M. Induction of microsomal dimethylnitrosamine demethylase by pyrazole. Biochem Pharmacol. 1982 Apr 1;31(7):1245–1249. doi: 10.1016/0006-2952(82)90011-9. [DOI] [PubMed] [Google Scholar]
- Golden S., Katz J. The determination of reduced nicotinamide-adenine dinucleotide and metabolic intermediates in picomole amounts with bacterial luciferase. Biochem J. 1980 Jun 15;188(3):799–805. doi: 10.1042/bj1880799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Hensgens L. A., Nieuwenhuis B. J., van der Meer R., Meijer A. J. The role of hydrogen translocating shuttles during ethanol oxidation in hepatocytes from euthyroid and hyperthyroid rats. Eur J Biochem. 1980;108(1):39–45. doi: 10.1111/j.1432-1033.1980.tb04693.x. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Lardy H. A. Effects of thyroid states on the Cori cycle, glucose--alanine cycle, and futile cycling of glucose metabolism in rats. Arch Biochem Biophys. 1981 Jun;209(1):41–51. doi: 10.1016/0003-9861(81)90254-x. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Hers H. G. Utile and futile cycles in the liver. Biochem Biophys Res Commun. 1974 Jun 4;58(3):540–548. doi: 10.1016/s0006-291x(74)80454-7. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L., Morré D. J. Distribution of phospholipid biosynthetic enzymes among cell components of rat liver. J Biol Chem. 1978 Nov 10;253(21):7960–7971. [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Rognstad R. Glucose phosphorylation, glucose-6-phosphatase, and recycling in rat hepatocytes. J Biol Chem. 1978 Jul 10;253(13):4530–4536. [PubMed] [Google Scholar]
- Kneer N. M., Wagner M. J., Lardy H. A. Regulation by calcium of hormonal effects on gluconeogenesis. J Biol Chem. 1979 Dec 10;254(23):12160–12168. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lake B. G., Collins M. A., Harris R. A., Phillips J. C., Cottrell R. C., Gangolli S. D. Studies on the metabolism of dimethylnitrosamine in vitro by rat-liver preparations. I. Comparison with mixed-function oxidase enzymes. Xenobiotica. 1982 Jul;12(7):435–445. doi: 10.3109/00498258209052485. [DOI] [PubMed] [Google Scholar]
- Landau B. R., Bartsch G. E. Estimations of pathway contributions to glucose metabolism and the transaldolase reactions. J Biol Chem. 1966 Feb 10;241(3):741–749. [PubMed] [Google Scholar]
- Lijinsky W., Loo J., Ross A. E. Mechanism of alkylation of nucleic acids by nitrosodimethylamine. Nature. 1968 Jun 22;218(5147):1174–1175. doi: 10.1038/2181174b0. [DOI] [PubMed] [Google Scholar]
- Lijinsky W., Reuber M. D., Manning W. B. Potent carcinogenicity of nitrosodiethanolamine in rats. Nature. 1980 Dec 11;288(5791):589–590. doi: 10.1038/288589a0. [DOI] [PubMed] [Google Scholar]
- Litterst C. L. Prolonged depression of hepatic microsomal drug metabolism and hemoprotein levels following a single dose of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU). Biochem Pharmacol. 1981 May 1;30(9):1014–1016. doi: 10.1016/0006-2952(81)90050-2. [DOI] [PubMed] [Google Scholar]
- Longenecker J. P., Williams J. F. Use of [2-14C]glucose and [5-14C]glucose for evaluating the mechanism and quantitative significance of the 'liver-cell' pentose cycle. Biochem J. 1980 Jun 15;188(3):847–857. doi: 10.1042/bj1880847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGEE P. N., BARNES J. M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br J Cancer. 1956 Mar;10(1):114–122. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. N., Barnes J. M. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163–246. doi: 10.1016/s0065-230x(08)60079-2. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- McLean A. E., Day P. A. The use of new methods to measure: the effect of diet and inducers of microsomal enzyme synthesis on cytochrome P-450 in liver homogenates, and on metabolism of dimethyl nitrosamine. Biochem Pharmacol. 1974 Apr 1;23(7):1173–1180. doi: 10.1016/0006-2952(74)90291-3. [DOI] [PubMed] [Google Scholar]
- Nilsson O. S., Arion W. J., Depierre J. W., Dallner G., Ernster L. Evidence for the involvement of a glucose-6-phosphate carrier in microsomal glucose-6-phosphatase activity. Eur J Biochem. 1978 Jan 16;82(2):627–634. doi: 10.1111/j.1432-1033.1978.tb12059.x. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Sukalski K. A., Alvares F. L. Responses of glucose 6-phosphate levels to varied glucose loads in the isolated perfused rat liver. J Biol Chem. 1980 Mar 10;255(5):1834–1838. [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Okajima F., Katz J. Effect of mercaptopicolinic acid and of transaminase inhibitors on glycogen synthesis by rat hepatocytes. Biochem Biophys Res Commun. 1979 Mar 15;87(1):155–162. doi: 10.1016/0006-291x(79)91660-7. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979 Aug 15;182(2):565–575. doi: 10.1042/bj1820565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesser T., Wurster B., Hess B. Determination of the kinetic constants of glucosephosphate isomerase by non-linear optimization. Isomerization and anomerization function. Eur J Biochem. 1979 Jul;98(1):93–98. doi: 10.1111/j.1432-1033.1979.tb13165.x. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Lake B. G., Phillips J. C., Gangolli S. D. Substrates and inhibitors of hepatic amine oxidase inhibit dimethylnitrosamine-induced mutagenesis in Salmonella typhimurium. Mutat Res. 1980 Aug;72(1):63–72. doi: 10.1016/0027-5107(80)90221-3. [DOI] [PubMed] [Google Scholar]
- SCHAMBYE P., WOOD H. G., POPJAK G. Biological asymmetry of glycerol and formation of asymmetrically labeled glucose. J Biol Chem. 1954 Feb;206(2):875–882. [PubMed] [Google Scholar]
- Schoental R., Gibbard S. Increased excretion of urinary porphyrins by white rats given intragastrically the chemical carcinogens diethylnitrosamine, monocrotaline, T-2 toxin and ethylmethanesulphonate [proceedings]. Biochem Soc Trans. 1979 Feb;7(1):127–129. doi: 10.1042/bst0070127. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. A sensitive and simple method for the study of oxaloacetate compartmentation in isolated hepatocytes. FEBS Lett. 1976 Nov;70(1):51–55. doi: 10.1016/0014-5793(76)80724-7. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Decarboxylation of porphyrinogens by rat liver uroporphyrinogen decarboxylase. Biochem J. 1979 Nov 1;183(2):455–458. doi: 10.1042/bj1830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuckler E. A., Arrhenius E., Hultin T. Alterations in microsomal electron transport, oxidative N-demethylation and azo-dye cleavage in carbon tetrachloride and dimethylnitrosamine-induced liver injury. Biochem J. 1967 Apr;103(1):55–64. doi: 10.1042/bj1030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochor M., Baquer N. Z., McLean P. Regulation of pathways of glucose metabolism in kidney. The effect of experimental diabetes on the activity of the pentose phosphate pathway and the glucuronate-xylulose pathway. Arch Biochem Biophys. 1979 Dec;198(2):632–646. doi: 10.1016/0003-9861(79)90541-1. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Blum J. J. Quantitative analysis of intermediary metabolism in Tetrahymena pyriformis. Cells kept under static conditions for four hours after growth in glucose-supplemented medium. J Biol Chem. 1981 Mar 25;256(6):2752–2760. [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem J. 1976 Oct 1;159(1):7–14. doi: 10.1042/bj1590007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., De Matteis F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem J. 1979 Apr 15;180(1):241–244. doi: 10.1042/bj1800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Study of the fructose 6-phosphate/fructose 1,6-bi-phosphate cycle in the liver in vivo. Biochem J. 1980 Oct 15;192(1):263–271. doi: 10.1042/bj1920263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N., Argus M. F., Arcos J. C. Mechanism of 3-methylcholanthrene-induced inhibition of dimethylnitrosamine demethylase in rat liver. Cancer Res. 1970 Oct;30(10):2556–2562. [PubMed] [Google Scholar]
- WEBER G. STUDY AND EVALUATION OF REGULATION OF ENZYME ACTIVITY AND SYNTHESIS IN MAMMALIAN LIVER. Adv Enzyme Regul. 1963;1:1–35. doi: 10.1016/0065-2571(63)90004-9. [DOI] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- White I. N. Metabolic activation of acetylenic substituents to derivatives in the rat causing the loss of hepatic cytochrome P-450 and haem. Biochem J. 1978 Sep 15;174(3):853–861. doi: 10.1042/bj1740853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N., Muller-Eberhard U. Decreased liver cytochrome P-450 in rats caused by norethindrone or ethynyloestradiol. Biochem J. 1977 Jul 15;166(1):57–64. doi: 10.1042/bj1660057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. L., Larson R. E., Moldowan M. J. Effect of BCNU on antipyrine metabolism in mice. Proc West Pharmacol Soc. 1981;24:173–176. [PubMed] [Google Scholar]
- de Matteis F., Gibbs A. H., Jackson A. H., Weerasinghe S. Conversion of liver haem into N-substituted porphyrins or green pigments. Nature of the substituent at the pyrrole nitrogen atom. FEBS Lett. 1980 Sep 22;119(1):109–112. doi: 10.1016/0014-5793(80)81009-x. [DOI] [PubMed] [Google Scholar]


