Abstract
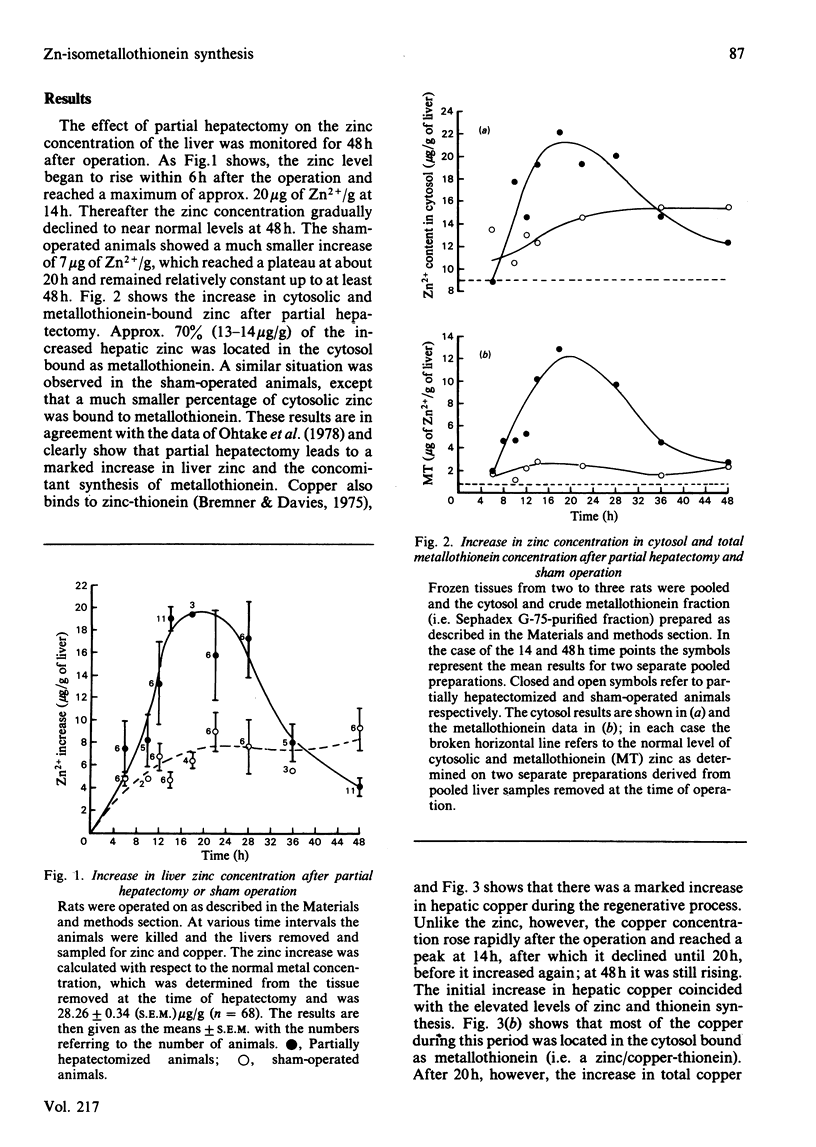
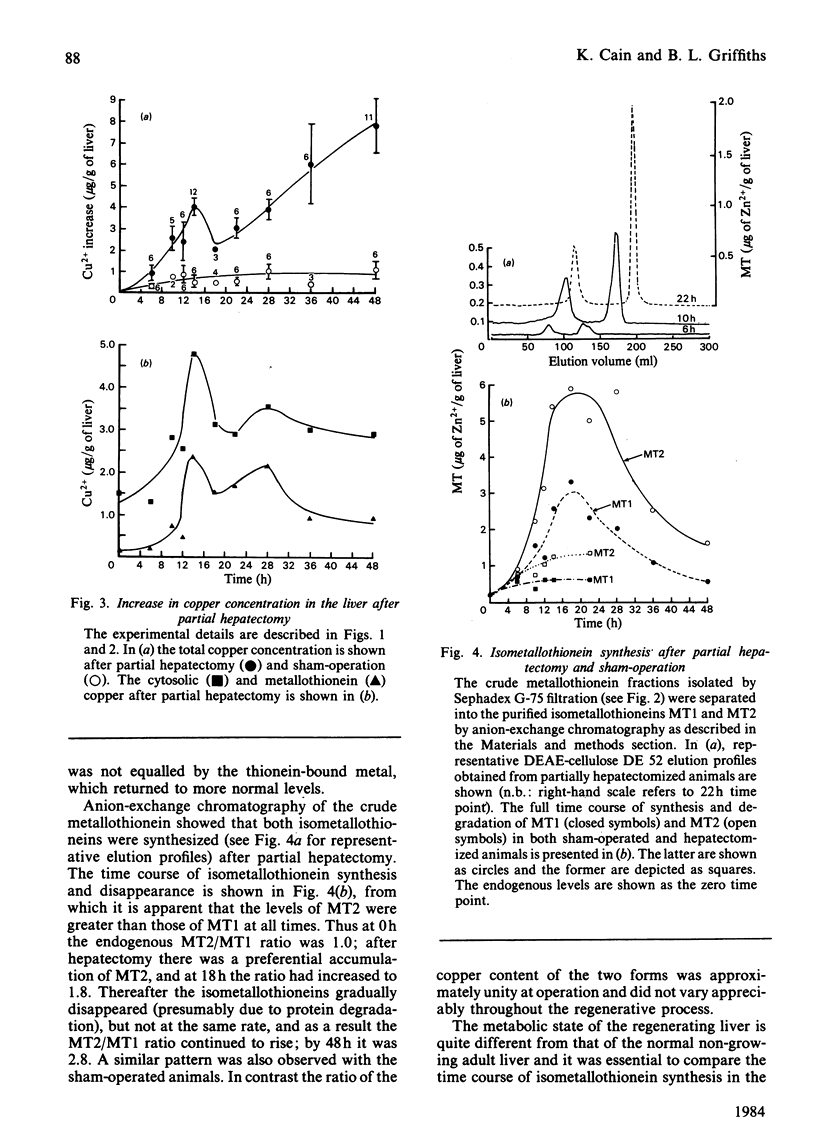
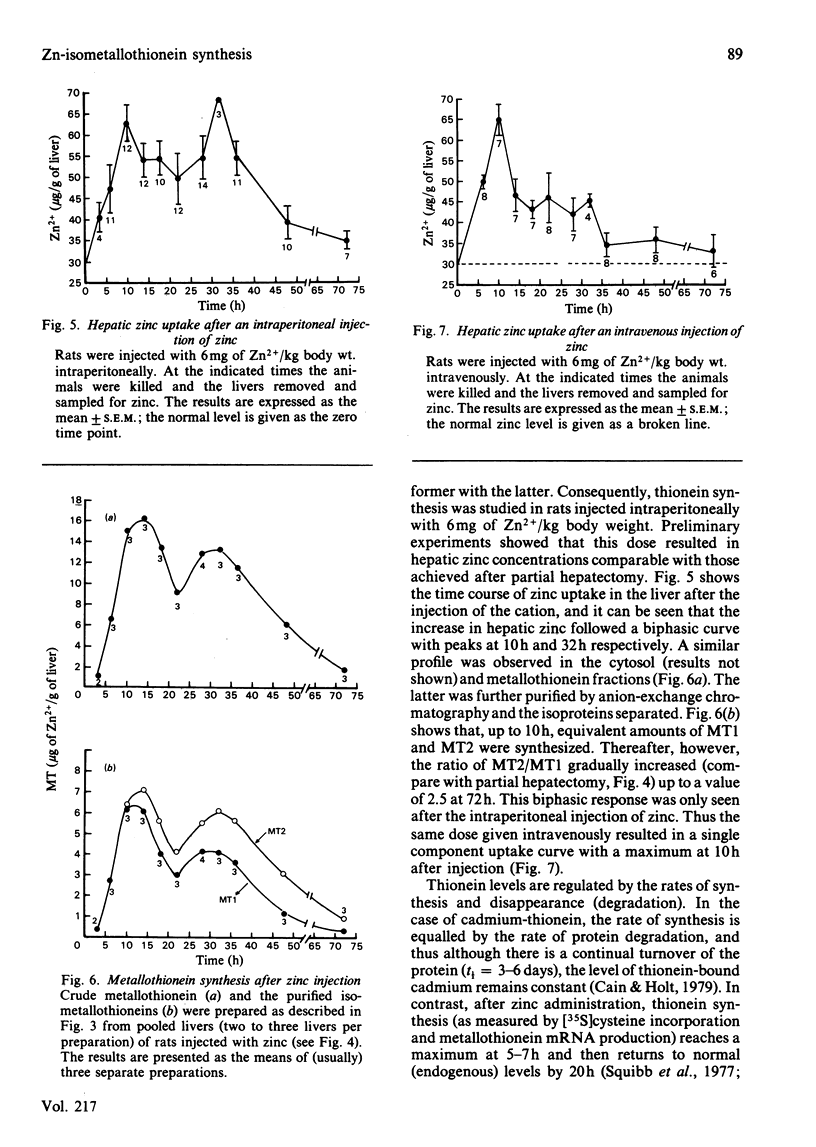
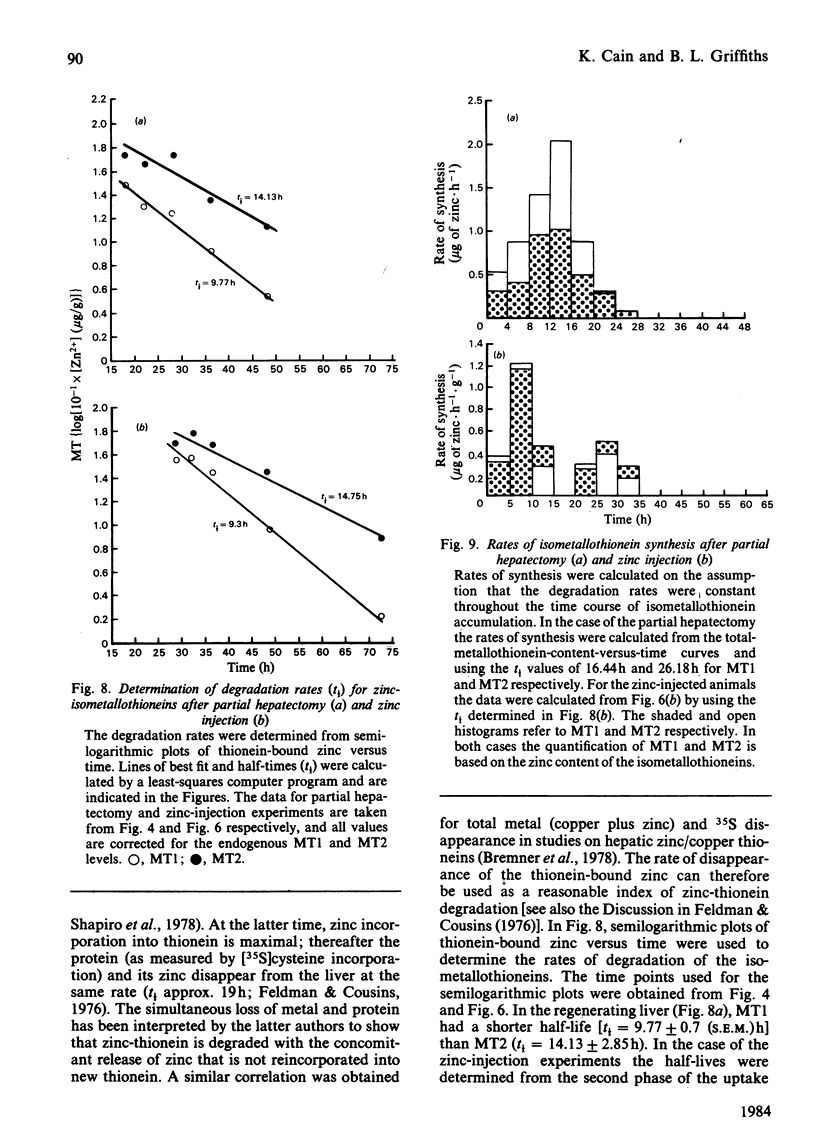
The time course of hepatic zinc-isometallothionein synthesis was studied in the regenerating liver and compared with that produced after the parenteral injection of zinc (6 mg of Zn2+/kg). In the regenerating liver, zinc levels rose rapidly after partial hepatectomy and reached a maximum at approx. 14h before declining to approximately normal levels at 48h post-operation. During this 48h period most of the zinc was incorporated into metallothionein. Purification of the latter into the charge-separable isometallothioneins (i.e. MT1 and MT2) showed that, in the regenerating liver, there was an unequal distribution of zinc between the two isoproteins. Thus at operation the endogenous thionein had an MT2/MT1 ratio of 1; after regeneration this ratio increased, and all times during the time course there was more MT2 than MT1. In contrast, the intraperitoneal injection of zinc produced a biphasic uptake of zinc into the liver with maxima at 10h and 32h. During the first phase of zinc uptake, metallothionein synthesis increased rapidly and, unlike the regenerating liver, the MT2/MT1 ratio of 1 remained constant. Thereafter, this ratio increased in a manner analogous to that exhibited by the regenerating liver. Half-life determinations for thionein disappearance/degradation shows that MT2 and MT1 were degraded with half-lives (t1/2) of 26.18h and 16.44h respectively in the regenerating liver and 14.75h and 9.3h after zinc injection. Thus thionein disappearance/degradation in the regenerating liver was slower than that seen after zinc injection. However, in both situations MT2 was always removed at a slower rate than MT1. Calculation of the rates of thionein synthesis (assuming the above disappearance rates were constant throughout the time course) showed that, in the regenerating liver, the rate of MT2 synthesis was approximately twice that of MT1. This was not the case after zinc injection, where both isometallothioneins were synthesized in equal amounts. These results demonstrate that the rates of synthesis of MT2 and MT1 can be altered according to the metabolic status of the cell and suggest a specific role for MT2 during liver regeneration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Winter W. P., Maher J. J., Bernstein I. A. Turnover of metallothioneins in rat liver. Biochem J. 1978 Jul 15;174(1):327–338. doi: 10.1042/bj1740327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. U. Induction of hepatic metallothionein in the immature rat following administration of cadmium. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):148–155. doi: 10.1016/0041-008x(80)90015-0. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Hoekstra G., Davies N. T., Young B. W. Effect of zinc status of rats on the synthesis and degradation of copper-induced metallothioneins. Biochem J. 1978 Sep 15;174(3):883–892. doi: 10.1042/bj1740883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Holt D. E. Metallothionein degradation: metal composition as a controlling factor. Chem Biol Interact. 1979;28(1):91–106. doi: 10.1016/0009-2797(79)90117-0. [DOI] [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R., Brady F. O., Webb M. Metabolism of zinc and copper in the neonate: accumulation of Cu in the gastrointestinal tract of the newborn rat. Br J Nutr. 1981 Mar;45(2):391–399. doi: 10.1079/bjn19810114. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Sadler P. J., Cain K., Holt D. E., Webb M., Hawkes G. E. 88MHz 113Cd-n.m.r. studies of native rat liver metallothioneins. Biochem J. 1983 Apr 1;211(1):251–255. doi: 10.1042/bj2110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H., Hasegawa K., Koga M. Zinc-binding protein in the livers of neonatal, normal and partially hepatectomized rats. Biochem J. 1978 Sep 15;174(3):999–1005. doi: 10.1042/bj1740999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H., Koga M. Purification and characterization of zinc-binding protein from the liver of the partially hepatectomized rat. Biochem J. 1979 Dec 1;183(3):683–690. doi: 10.1042/bj1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISCATOR M. OM KADMIUM I NORMALA MAENNISKONJURAR SAMT REDOGOERELSE FOER ISOLERING AV METALLOTHIONEIN UR LEVER FRAN KADMIUMEXPONERADE KANINER. Nord Hyg Tidskr. 1964;45:76–82. [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Influence of parenteral zinc and actinomycin D on tissue zinc uptake and the synthesis of a zinc - binding protein. Bioinorg Chem. 1975 Apr;4(3):215–224. doi: 10.1016/s0006-3061(00)80104-0. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Shapiro S. G., Squibb K. S., Markowitz L. A., Cousins R. J. Cell-free synthesis of metallothionein directed by rat liver polyadenylated messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):833–840. doi: 10.1042/bj1750833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squibb K. S., Cousins R. J., Feldman S. L. Control of zinc-thionein synthesis in rat liver. Biochem J. 1977 Apr 15;164(1):223–228. doi: 10.1042/bj1640223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M. Induction of zinc-thionein in rat liver and kidneys by zinc loading as studied at isometallothionein levels. Toxicol Lett. 1980 Jun;6(1):59–65. doi: 10.1016/0378-4274(80)90103-4. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M., Cain K. Functions of metallothionein. Biochem Pharmacol. 1982 Jan 15;31(2):137–142. doi: 10.1016/0006-2952(82)90202-7. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Differences in the polymorphic forms of metallothionein. Arch Biochem Biophys. 1982 Mar;214(1):80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]


