Abstract
Purpose
Although the association between appendicitis and colorectal cancer in older patients has received attention, postoperative colorectal screening through endoscopy is not currently recommended. This study conducted a systematic review of the literature on colorectal screening following appendectomy in adult patients.
Methods
A literature search was performed using online databases. Studies reporting colorectal surveillance after appendectomy in adult patients were retrieved for assessment.
Results
Eight articles including a total of 3,995 patients were published between 2013 and 2023. An age of 40 years was the lower threshold in 6 of the 8 articles. Postoperative colorectal screening occurred in 771 patients (19.3%). Endoscopy was performed in 95.2% of cases and computed tomography–colonography in 4.8%. During endoscopic examinations, a lesion was discovered in 184 of 771 patients (24.0%), and an adenomatous polyp was found in 154 of 686 patients (22.5%). The overall cancer rate was 3.9% (30 of 771 patients). The tumor was located in the right-sided colon in 46.7% of the patients, in the cecum in 20.0%, in the rectum in 16.7%, in the left-sided colon in 10.0%, and in the sigmoid colon in 6.7%.
Conclusion
Performing post-appendectomy colorectal screening in patients >40 years of age could allow early detection of an underlying lesion.
Keywords: Appendicitis, Colorectal neoplasms, Screening, Endoscopy
INTRODUCTION
Appendectomy is one of the most common surgical procedures performed in an emergency setting by general surgeons. The peak incidence of acute appendicitis occurs between the ages of 15 and 30 years, with a gradual decrease thereafter [1].
The correlation between cancer and appendicitis in adult patients is unclear. In a large cohort study, Wu et al. [2] showed that after appendectomy, the incidence rate of any type of cancer was 4.64 times higher than in patients without appendectomy; furthermore, colorectal cancer was the second most common type, and the cancer risk was highest in the first 3 months after surgery and declined during the following months. In another retrospective analysis [3], the odds ratio of colon cancer incidence was 38.5 in patients >40 years of age who underwent appendectomy. Compared to the general population, appendectomy patients past the age of 40 years were found to be at a 10-fold elevated risk of right-sided colon cancer [4].
Acute appendicitis could therefore be an early sign of simultaneous underlying colorectal cancer. The pathophysiological mechanism is not yet well known but could be related to pseudo-obstruction of the appendiceal lumen [5]. Furthermore, immune-mediated lymphoid hyperplasia resulting from a cancerous lesion could lead to obstruction of the appendiceal lumen, causing appendicitis [6, 7].
Thus, the dilemma arises of whether it is justifiable and necessary to perform more invasive investigations in adult appendectomy patients during the postoperative period. Currently, there are no formal recommendations regarding the need for endoscopic surveillance after an episode of acute appendicitis, and adult patients are usually treated in the same manner as younger ones.
The aim of this systematic review was to provide an exhaustive analysis of the available literature regarding the utility of colorectal screening after appendectomy in adult patients.
METHODS
This systematic review was performed following an open protocol registered with the PROSPERO (International Prospective Register of Systematic Reviews; No. CRD42023441767), and the results were reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [8].
Study inclusion criteria
For proper identification of studies eligible for analysis, the study selection criteria were defined before data collection was initiated. All studies that included patients aged >40 years who received a colorectal screening following an appendectomy were eligible for inclusion. Articles investigating the rate of colorectal tumors incidentally discovered in appendectomies without surveillance colonoscopy were not considered.
Types of study
Case-control studies, case series, and case reports were considered appropriate for this systematic review. Review articles, meta-analyses, conference abstracts, letters, and commentaries were not considered.
Types of participants and types of intervention
Adult patients (>40 years) who had received a colonoscopy in the months following an appendectomy for acute appendicitis were considered. Patients who were treated with nonoperative management for acute appendicitis with delayed surgery and subsequent endoscopic surveillance were also included.
Types of outcome measures
The primary outcomes were the incidence rates of colorectal cancer and adenomatous polyps discovered during endoscopic investigation after the appendectomy. All secondary parameters reported in the selected studies (e.g., malignancy suspected based on a computed tomography [CT] scan, nonoperative management rate, colonoscopy rate) were also evaluated.
Literature search strategy
A literature search was performed on the following online databases: MEDLINE (via PubMed), Embase, and Google Scholar. To increase the probability of identifying all relevant articles, a specific research equation was formulated for each database, using the following keywords and MeSH (Medical Subject Headings) terms: appendicitis, appendix, acute appendicitis, colorectal neoplasm, colorectal, colorectal cancer, colorectal carcinoma, colonoscopy, and endoscopic surveillance.
In addition, the reference lists from the eligible studies and relevant review articles (not included in the systematic review) were cross-checked to identify additional records. The literature search was performed in May 2023, and no time limit was applied. Only studies that were written in English, French, or Italian and that met the selection criteria were reviewed.
Study selection and quality assessment
Two reviewers (FE and MDP) independently screened the titles and abstracts of the retrieved studies for relevance. To enhance sensitivity, records were removed only if both reviewers excluded the record during the title screening stage. All disagreements were resolved based on discussions with a third reviewer (AC). Subsequently, both primary reviewers performed a full-text analysis of the selected articles independently and assessed the risks of bias and study quality using the GRADE (Grading of Recommendations Assessment Development and Evaluation) system [9].
Data extraction
Data extracted from the studies included in the systematic review were processed for qualitative and, if appropriate, quantitative analyses. Outcome measures (mean and median values, standard deviation, and ranges) were extracted for each variable. The average post appendicectomy colorectal screening rate, the incidence rate of colorectal cancer, and the incidence rate of adenomatous polyps were calculated, where relevant.
RESULTS
Study and patients’ characteristics
From the 952 articles initially identified, 8 articles [5, 10–16] met the inclusion criteria and were selected for the systematic review. Fig. 1 shows the flow chart of how studies were identified and then included or excluded. The selected articles consisted of 8 single-center case series [5, 10–16] that were published between 2013 and 2023 and carried out in 5 different nations: Australia [5, 10, 14], New Zealand [11], United Kingdom [12, 13], Norway [16], and Ireland [15].
Fig. 1.
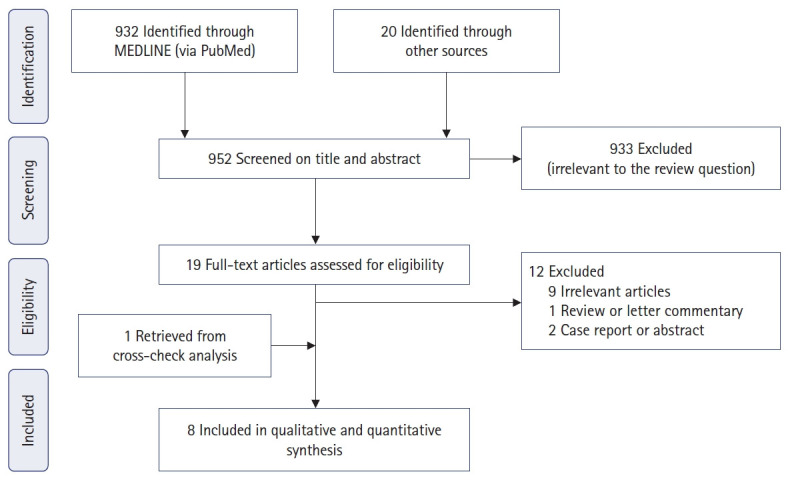
Flowchart of study search, selection, and inclusion.
Overall, 3,995 patients were included in this systematic review (Table 1) [5, 10–16]. The cutoff age considered was 40 years old in 6 articles [10–13, 15, 16], 45 years in 1 article [5], and 50 years in 1 article [14]. The age range was between 40 and 96 years, and 51.0% (range, 43.8%–53.0%) were men. Appendicitis was diagnosed in 68.3% (range, 41.8%–88.1%) by CT scan, and a suspected tumor was mentioned in 2.0% of the patients undergoing preoperative CT scans. In the study population, 94.7% of patients underwent an emergency appendectomy, and 5.3% were initially chosen for nonoperative management (Table 2) [5, 10–16]. Postoperative colorectal screening occurred in 771 of 3,995 patients (19.3%). Of these, in 95.2% of cases a colonoscopy was performed, and in 4.8% a CT-colonography took place. Colorectal screening was completed between 3.5 and 60 months after an appendectomy.
Table 1.
Demographic and clinical data of studies
| Study | Type | Study period | Age (yr) |
No. of participants (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Cutoff | Median (range) | Total | Male sex | CT scan prior to surgery | CT scan suspected malignancy | |||
| Jolly et al. [10] (2023) | Single-center retrospective study | 2015–2022 | >40 | 54 (40–92) | 176 (100) | 77 (43.8) | 155 (88.1) | NA |
| Lee et al. [11] (2023) | Single-center retrospective study | 2010–2015 | >40 | NA | 1,099 (100) | 571 (52.0) | 831 (75.6) | 7 (8.4) |
| Seretis et al. [12] (2020) | Single-center retrospective study | 2016–2019 | >40 | 61.2 (41–88) | 83 (100) | 44 (53.0) | 66 (79.5) | 0 (0) |
| Mohamed et al. [13] (2019) | Single-center retrospective study | 2004–2014 | >40 | 52 (40–96) | 1,055 (100) | 546 (51.8) | NA | NA |
| Sylthe Pedersen et al. [16] (2018) | Single-center retrospective study | 2010–2015 | >40 | 54 (40–94) | 731 (100) | 358 (49.0) | 575 (78.7) | 23 (4.0) |
| Dhadlie and Mehanna [14] (2018) | Single-center retrospective study | 2017–2018 | >50 | 62 (50–85) | 43 (100) | 20 (46.5) | 37 (86.0) | 0 (0) |
| Shine et al. [5] (2017) | Single-center retrospective study | 2003–2015 | ≥45 | 57 (45–97) | 629 (100) | 330 (52.5) | 263 (41.8) | NA |
| Khan et al. [15] (2013) | Single-center retrospective study | 2005–2011 | >40 | 54 (40–89) | 179 (100) | 92 (52.0) | 80 (44.7) | 1 (1.3) |
| Total | - | - | - | 40–96 | 3,995 (100) | 2,038 (51.0) | 2,007 (68.3) | 31 (2.0) |
CT, computed tomography; NA, not available.
Table 2.
Patients’ characteristics
| Study | No. of participants |
Time to colonoscopy (mo) | |||
|---|---|---|---|---|---|
| Total | Surgery | Nonoperative management | Endoscopic surveillance | ||
| Jolly et al. [10] (2023) | 176 (100) | 163 (92.6) | 11 (6.3) | 100 (56.8) | 3.5 |
| Lee et al. [11] (2023) | 1,099 (100) | 1,054 (95.9) | 46 (4.2) | 210 (19.1)a | <36 |
| Seretis et al. [12] (2020) | 83 (100) | 83 (100) | 0 (0) | 14 (16.9) | NA |
| Mohamed et al. [13] (2019) | 1,055 (100) | 1,055 (100) | 0 (0) | 26 (2.5)b | NA |
| Sylthe Pedersen et al. [16] (2018) | 731 (100) | 616 (84.3) | 115 (15.7) | 316 (43.2) | <36 |
| Dhadlie and Mehanna [14] (2018) | 43 (100) | 43 (100) | 0 (0) | 20 (46.5) | NA |
| Shine et al. [5] (2017) | 629 (100) | 593 (94.6) | 36 (5.4) | 74 (11.8) | 8 |
| Khan et al. [15] (2013) | 179 (100) | 179 (100) | 0 (0) | 11 (6.1) | <60 |
| Total | 3,995 (100) | 3,785 (94.7) | 208 (5.3) | 771 (19.3) | 3.5–60 |
NA, not available; CT, computed tomography.
Including 30 cases where CT-colonography was performed.
Including 7 cases where CT-colonography was performed.
Colorectal screening results
During the endoscopic examinations, a lesion was found in 184 of 771 patients (23.9%); the lesion was an adenomatous polyp in 83.7% of these cases and a tumor in 16.3%. Regarding the polyps, 28.6% were found in the cecum, 12.2% in the right-sided colon, 14.3% in the transverse colon, 2.0% in the left-sided colon, 26.5% in the sigmoid colon, and 16.3% in the rectum (Table 3) [5, 10–16].
Table 3.
Colorectal screening results
| Study | No. of patients (%) |
|||
|---|---|---|---|---|
| Cancer detected through colonoscopy |
Adenomatous polyp detected |
|||
| Total | Colorectal location | Total | Colorectal location | |
| Jolly et al. [10] (2023) (n=100) | 0 (0) | NA | 15 (15.0) | Cecum: 3 (20.0) |
| Transverse: 4 (26.7) | ||||
| Sigmoid: 4 (26.7) | ||||
| Rectum: 4 (26.7) | ||||
| Lee et al. [11] (2023) (n=210) | 11 (5.2) | Right: 8 (72.7) | 90 (42.9) | NA |
| Left: 2 (18.2) | ||||
| Rectum: 1 (9.1) | ||||
| Seretis et al. [12] (2020) (n=14) | 0 (0) | NA | 2 (14.3) | Right: 2 (100) |
| Mohamed et al. [13] (2019) (n=26) | 2 (7.7) | Right: 2 (100) | 1 (3.9) | Right: 1 (100) |
| Sylthe Pedersen et al. [16] (2018) (n=316) | 9 (2.9) | Cecum: 4 (44.4) | 45 (14.2) | Cecum: 11 (24.4) |
| Right: 3 (33.3) | Right: 3 (6.7) | |||
| Rectum: 2 (22.2) | Transverse: 2 (4.4) | |||
| Left: 1 (2.2) | ||||
| Sigmoid: 9 (20.0) | ||||
| Rectum: 4 (8.9) | ||||
| Dhadlie and Mehanna [14] (2018) (n=20) | 1 (5.0) | Right: 1 (100) | 1 (5.0) | Transverse: 1 (100) |
| Shine et al. [5] (2017) (n=74) | 5 (6.8) | Cecum: 2 (40.0) | NA | NA |
| Left: 1 (20.0) | ||||
| Sigmoid: 1 (20.0) | ||||
| Rectum: 1 (20.0) | ||||
| Khan et al. [15] (2013) (n=11) | 2 (1.8) | Sigmoid: 1 (50.0) | NA | NA |
| Rectum: 1 (50.0) | ||||
| Total (n=771) | 30 (3.9) | Cecum: 6 (20.0) | 154/686 (22.5) | Cecum: 14/49 (28.6) |
| Right: 14 (46.7) | Right: 6/49 (12.2) | |||
| Left: 3 (10.0) | Transverse: 7/49 (14.3) | |||
| Sigmoid: 2 (6.7) | Left: 1/49 (2.0) | |||
| Rectum: 5 (16.7) | Sigmoid: 13/49 (26.5) | |||
| Rectum: 8/49 (16.3) | ||||
NA, not available.
In 30 of the 771 patients, cancer was diagnosed as a result of colorectal endoscopic screening. The overall cancer rate was 3.9% (range, 0%–7.7%). Tumors were found in the cecum in 20.0% of the cases, in the right-sided colon in 46.7%, in the left-sided colon in 10.0%, in the sigmoid colon in 6.7%, and in the rectum in 16.7% (Fig. 2, Table 3) [5, 10–16].
Fig. 2.
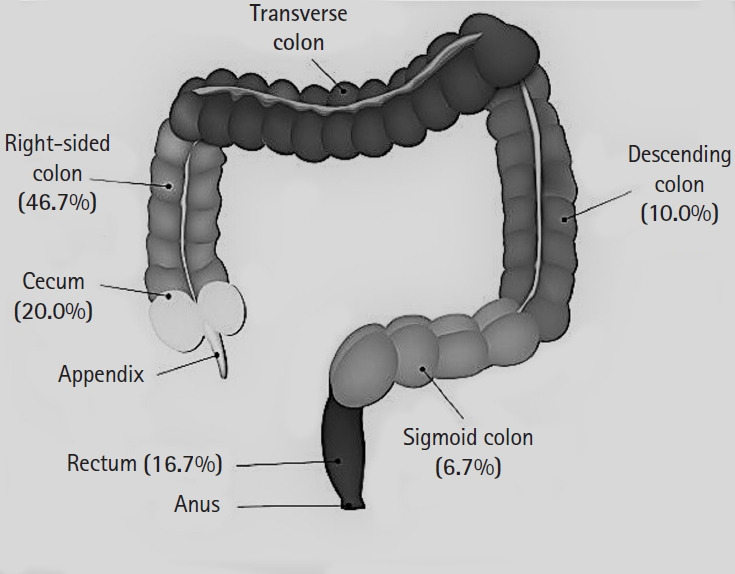
Colorectal cancer locations.
Among the 30 patients diagnosed with cancer, 7 cases were already suspected through preoperative CT scans. Postoperative screening allowed the identification of 23 cases of unsuspected tumors, and the adjusted cancer rate, excluding the cases found through preoperative CT scans, was therefore 3.0% (23 of 771 patients).
Study quality assessment
Based on the GRADE system [17], all 8 studies [5, 10–16] were of very low quality due to the absence of randomized controlled trials, the inclusion of only case series, a high risk of bias, and insufficient information about how the included patients were selected.
DISCUSSION
In this systematic review of the literature, we found that through colorectal screening after appendectomy, 1 in 4 patients over age 40 years were diagnosed with a colorectal lesion and, of these, 16.3% were cancerous. Our results suggested that this category of patients would benefit from systematic postoperative colorectal screening to identify underlying lesions early.
In a recent literature review and meta-analysis including 8 observational studies and 4,328 patients, Hajibandeh et al. [4] showed that the risk of right-sided colon cancer in appendectomy patients >40 years was significantly higher than the risk in the general population (P<0.0001). The pooled incidence of right-sided colon cancer was 1.043%, and 112 patients had to be investigated postoperatively to detect 1 case of right-sided colon cancer. Considering only appendectomy patients >50 years of age, the pooled incidence of right-sided colon cancer grew to 1.854%, and the number of screenings necessary to detect 1 case of right-sided colon cancer was reduced to 66. This result could be even more important if the complete colon-rectum area were considered and not exclusively the right-sided colon. In our review, synchronous tumors were found in the right-sided colon or cecum in 67% of cases, and in the remaining 33%, the descending colon, sigmoid, or rectum was involved.
In a large population study, Wu et al. [18] examined the correlation between appendectomy and colorectal cancer development. In a 14-year follow-up, the incidence of colorectal cancer was 14% higher than in the comparison cohort (P<0.05), and the highest incidence was observed for rectal cancer and for subjects >60 years old. In the appendectomy group, colorectal cancer appeared earlier than in the reference cohort (1.5–3.5 years post appendectomy), with an incidence of 5.52 per 10,000 person-years and a hazard ratio of 2.13.
These results accord with our study’s findings. The growth of a tumor could occur in the months following an acute episode of appendicitis, and appendicitis could be an early sign of cancer. An associated precancerous lesion could already be present at the time of the acute episode. In our review of the literature, colonoscopy in the months following an appendectomy identified a lesion in 24% of the cases, and of these, 84% was an adenomatous polyp. This data could explain the higher incidence of cancer in the 1.5 to 3.5 years after an appendectomy. The incidence rate of advanced adenomatous lesions (22.5%) in our study is much higher than reported in the literature (about 7%–8%) for asymptomatic patients aged >55 years who undergo endoscopic screening [19]. The timing of screening is not yet well defined, and the studies included in our review performed endoscopy between 3.5 and 60 months after surgery. Wu et al. [2], in their large cohort study, showed that tumor incidence was higher in the first 3 months after appendectomy. For this reason, we believe that screening should be performed during this time interval.
Another topic of ongoing debate is the role of imaging in diagnosing acute appendicitis [20]. When acute appendicitis is suspected in adult patients, the World Society of Emergency Surgery (WSES) Jerusalem Guidelines [21] recommend conducting a clinical-biological assessment, including the appendicitis inflammatory response score and the adult appendicitis score, together with point-of-care ultrasonography. A CT scan should ideally be performed in cases with negative ultrasound findings [21]. Furthermore, low-dose CT scans should be preferred, since it is as effective as standard CT in detecting complicated forms of appendicitis in adult patients with a body mass index <30 kg/m2 [22]. The articles included in our review showed an overall preoperative CT scan rate of 68.3%, with a range from 41.8% to 88.0%. Colorectal cancer was suspected in 2% of patients overall, and of the 30 patients in whom cancer was diagnosed through endoscopy, 7 cases were suspected preoperatively. However, because these late patients were included by the studies’ authors in the total number of tumors discovered during screening, the overall cancer rate was 3.9% and the adjusted rate was 2.9%. In a retrospective analysis of an online registry of endoscopies performed on 269,000 patients aged >55 years, Bokemeyer et al. [19] reported a colorectal cancer incidence of 0.77%, which is 4 times lower than what we found in patients after appendectomy. The need to carefully examine the colon preoperatively and conduct a postoperative screening in adult patients would seem crucial for excluding an underlying neoplasm.
In our review, 5% of post-appendectomy screenings were performed by CT-colonography. Considerable evidence exists to suggest that CT-colonography is as accurate as a colonoscopy to identify colorectal cancer. It shows a high sensitivity, estimated at 90%, in detecting large polyps and adenomas >10 mm, but conversely, in the case of small lesions <9 mm, the sensitivity drops to 76% [23–25]. Because of the discomfort caused by bowel preparation, patients are reluctant to accept colonoscopy. An additional benefit of CT-colonography is its milder preparation, which increases the acceptance of the test [23]. CT-colonography could be offered as an alternative to colonoscopy in patients between 40 and 50 years of age without any risk factors.
This systematic review has several limitations. First, the literature is still very scarce. Second, the articles included were exclusively retrospective studies. Third, no included studies compared patients undergoing endoscopy with those not undergoing screening. Lastly, in some cases endoscopic screening took place as late as 60 months after appendectomy.
In conclusion, to detect any underlying colorectal lesions early, a 3-month postoperative endoscopic screening should be offered to patients older than 40 years who are admitted for acute appendicitis. CT-colonography could be an alternative to endoscopy in some patients aged 40 to 50 years. Additional controlled studies should be performed to confirm our inferences.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Acknowledgments
The authors thank Oriane Le Campion for reviewing the manuscript for data management and English revision of this article.
Author contributions
Conceptualization: FE; Data curation: FE, MDP, MM; Formal analysis: FE, MM; Investigation: FE, MM, FD; Methodology: FE, MDP, MM; Resources: AC; Supervision: AC; Validation: AC; Visualization: FD, AC; Writing–original draft: FE, MDP, FD; Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
- 1.Lin KB, Chan CL, Yang NP, Lai RK, Liu YH, Zhu SZ, et al. Epidemiology of appendicitis and appendectomy for the low-income population in Taiwan, 2003-2011. BMC Gastroenterol. 2015;15:18. doi: 10.1186/s12876-015-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu SC, Chen WT, Muo CH, Sung FC. Appendicitis as an early manifestation of subsequent malignancy: an Asian population study. PLoS One. 2015;10:e0122725. doi: 10.1371/journal.pone.0122725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai HW, Loong CC, Tai LC, Wu CW, Lui WY. Incidence and odds ratio of appendicitis as first manifestation of colon cancer: a retrospective analysis of 1873 patients. J Gastroenterol Hepatol. 2006;21:1693–6. doi: 10.1111/j.1440-1746.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 4.Hajibandeh S, Hajibandeh S, Morgan R, Maw A. The incidence of right-sided colon cancer in patients aged over 40 years with acute appendicitis: a systematic review and meta-analysis. Int J Surg. 2020;79:1–5. doi: 10.1016/j.ijsu.2020.04.065. [DOI] [PubMed] [Google Scholar]
- 5.Shine RJ, Zarifeh A, Frampton C, Rossaak J. Appendicitis presenting as the first manifestation of colorectal carcinoma: a 13-year retrospective study. N Z Med J. 2017;130:25–32. [PubMed] [Google Scholar]
- 6.Peltokallio P. Acute appendicitis associated with carcinoma of the colon. Dis Colon Rectum. 1966;9:453–6. doi: 10.1007/BF02617444. [DOI] [PubMed] [Google Scholar]
- 7.Gaetke-Udager K, Maturen KE, Hammer SG. Beyond acute appendicitis: imaging and pathologic spectrum of appendiceal pathology. Emerg Radiol. 2014;21:535–42. doi: 10.1007/s10140-013-1188-7. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly S, McCullough T, Gunning T, Maddern G, Wichmann M. The role of screening colonoscopy after appendicitis in patients over 40 years of age. ANZ J Surg. 2023;93:602–5. doi: 10.1111/ans.18325. [DOI] [PubMed] [Google Scholar]
- 11.Lee MS, Thomas A, Pearson JF, Purcell R, Frizelle F, Glyn T. Risk of colorectal cancer in patients with appendicitis over the age of 40 years. Colorectal Dis. 2023;25:624–30. doi: 10.1111/codi.16429. [DOI] [PubMed] [Google Scholar]
- 12.Seretis C, Gill J, Lim P, Archer L, Seretis F, Yahia S. Surveillance colonoscopy after appendicectomy in patients over the age of 40: targeted audit of outcomes and variability in practice. Chirurgia (Bucur) 2020;115:595–9. doi: 10.21614/chirurgia.115.5.595. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed I, Chan S, Bhangu A, Karandikar S. Appendicitis as a manifestation of colon cancer: should we image the colon after appendicectomy in patients over the age of 40 years? Int J Colorectal Dis. 2019;34:527–31. doi: 10.1007/s00384-018-03224-8. [DOI] [PubMed] [Google Scholar]
- 14.Dhadlie S, Mehanna D. Rates of colorectal cancer detection in screening colonoscopy post appendicectomy in patients 50 years and over. Ann Med Surg (Lond) 2018;36:239–41. doi: 10.1016/j.amsu.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SA, Khokhar HA, Nasr AR, Carton E. Incidence of right-sided colonic tumors (non-appendiceal) in patient’s ≥40 years of age presenting with features of acute appendicitis. Int J Surg. 2013;11:301–4. doi: 10.1016/j.ijsu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Sylthe Pedersen E, Stornes T, Rekstad LC, Martinsen TC. Is there a role for routine colonoscopy in the follow-up after acute appendicitis? Scand J Gastroenterol. 2018;53:1008–12. doi: 10.1080/00365521.2018.1485732. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence: study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Wu SC, Chen WT, Muo CH, Ke TW, Fang CW, Sung FC. Association between appendectomy and subsequent colorectal cancer development: an Asian population study. PLoS One. 2015;10:e0118411. doi: 10.1371/journal.pone.0118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bokemeyer B, Bock H, Hüppe D, Düffelmeyer M, Rambow A, Tacke W, et al. Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269000 cases. Eur J Gastroenterol Hepatol. 2009;21:650–5. doi: 10.1097/meg.0b013e32830b8acf. [DOI] [PubMed] [Google Scholar]
- 20.Sammalkorpi HE, Mentula P, Leppäniemi A. A new adult appendicitis score improves diagnostic accuracy of acute appendicitis: a prospective study. BMC Gastroenterol. 2014;14:114. doi: 10.1186/1471-230X-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Saverio S, Podda M, De Simone B, Ceresoli M, Augustin G, Gori A, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27. doi: 10.1186/s13017-020-00306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sippola S, Virtanen J, Tammilehto V, Grönroos J, Hurme S, Niiniviita H, et al. The accuracy of low-dose computed tomography protocol in patients with suspected acute appendicitis: the OPTICAP Study. Ann Surg. 2020;271:332–8. doi: 10.1097/SLA.0000000000002976. [DOI] [PubMed] [Google Scholar]
- 23.Obaro AE, Burling DN, Plumb AA. Colon cancer screening with CT colonography: logistics, cost-effectiveness, efficiency and progress. Br J Radiol. 2018;91:20180307. doi: 10.1259/bjr.20180307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Haan MC, van Gelder RE, Graser A, Bipat S, Stoker J. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: a meta-analysis. Eur Radiol. 2011;21:1747–63. doi: 10.1007/s00330-011-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


