Abstract
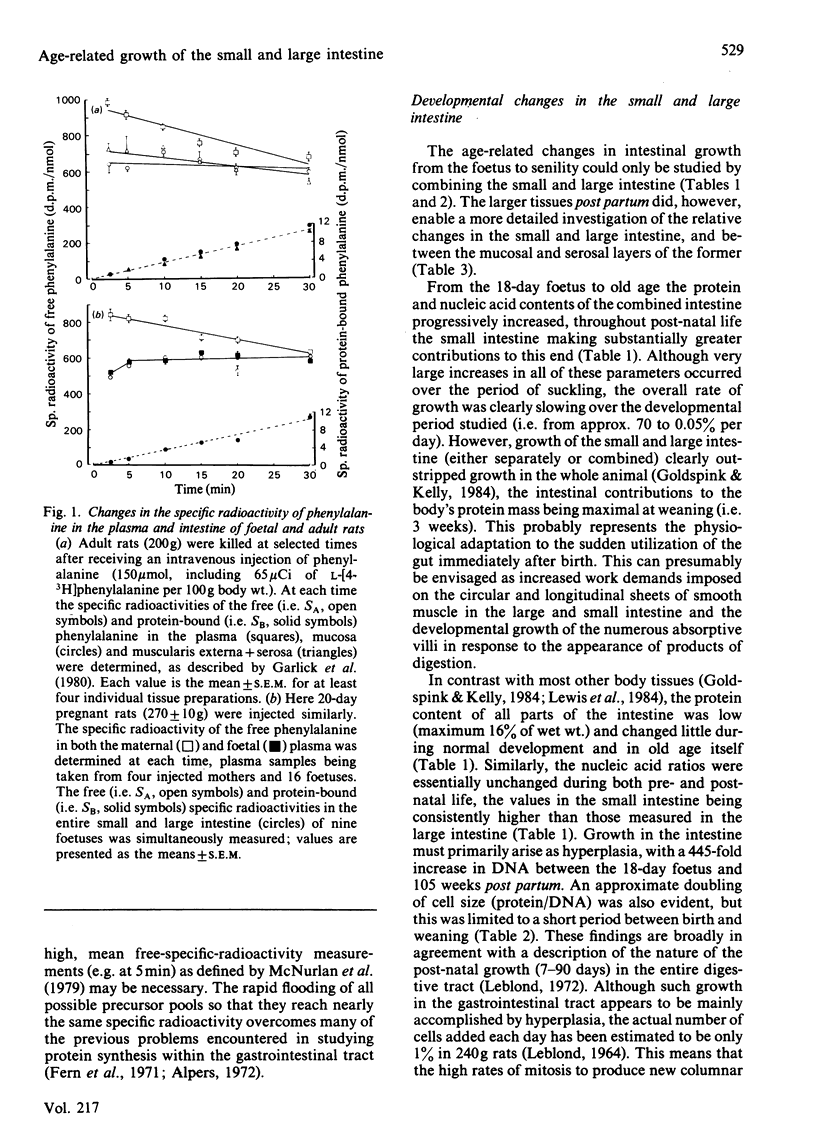
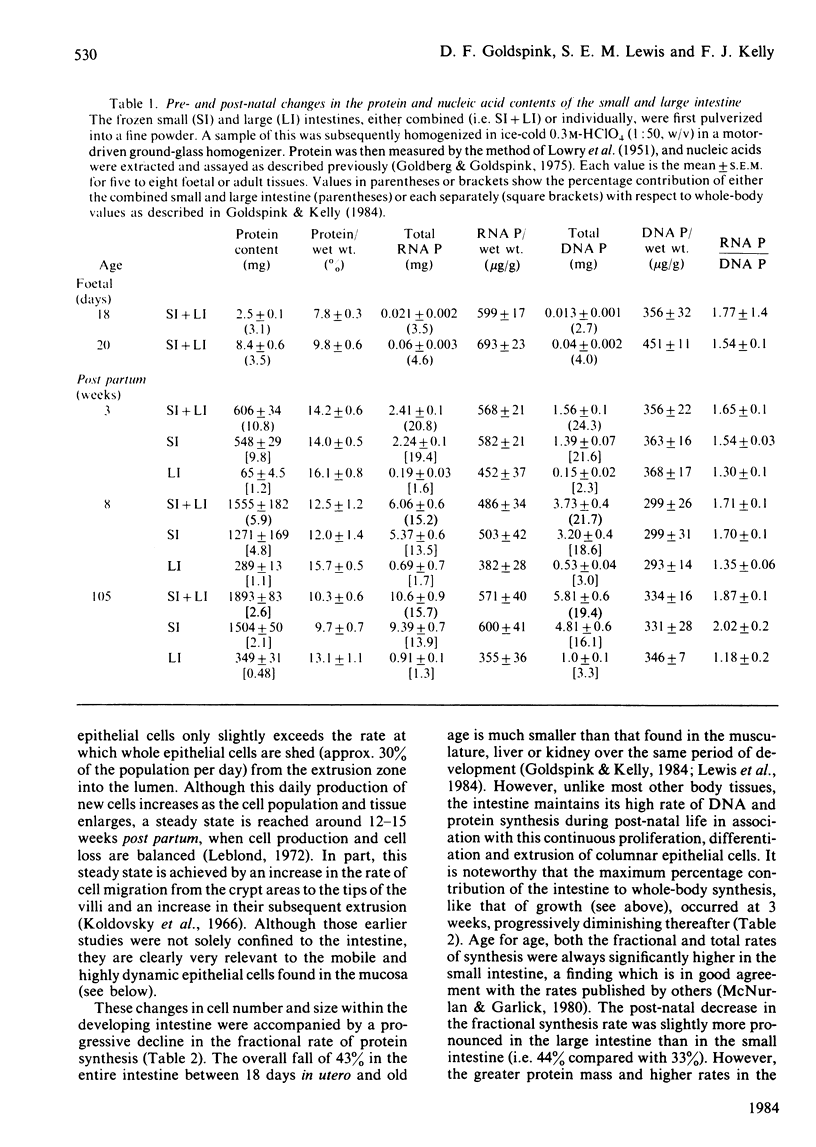
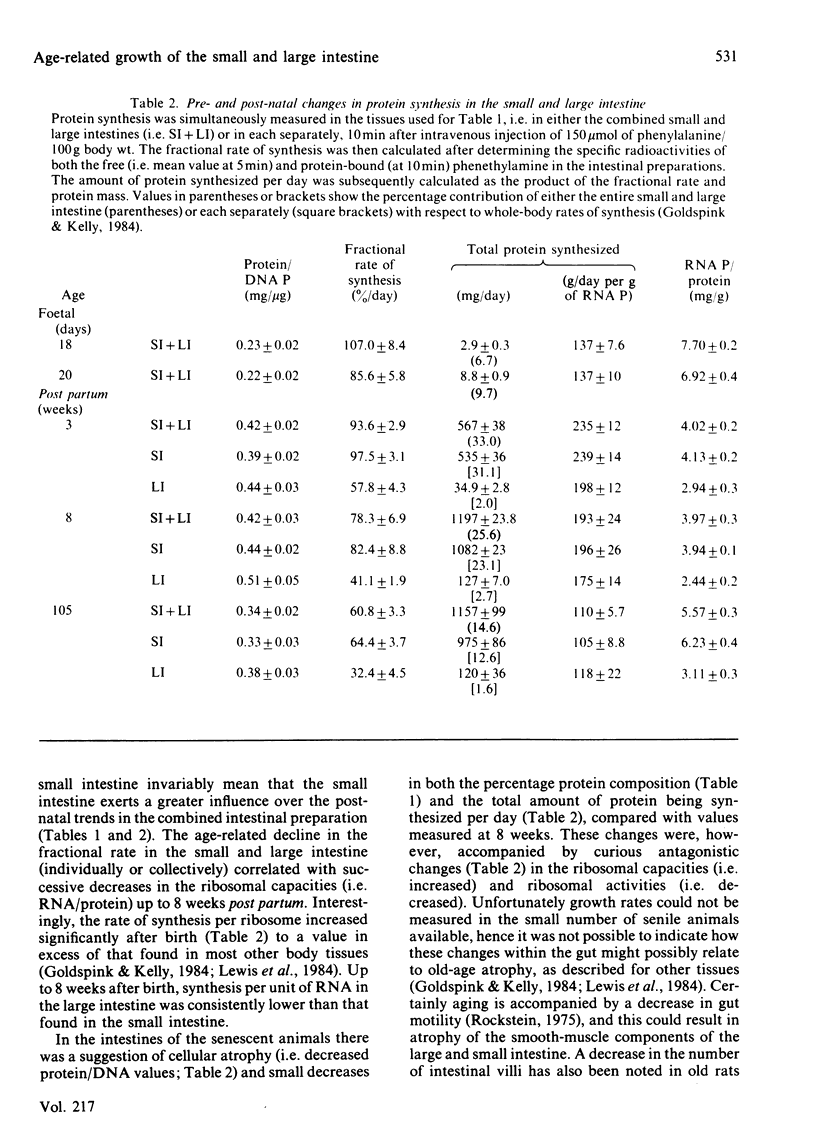
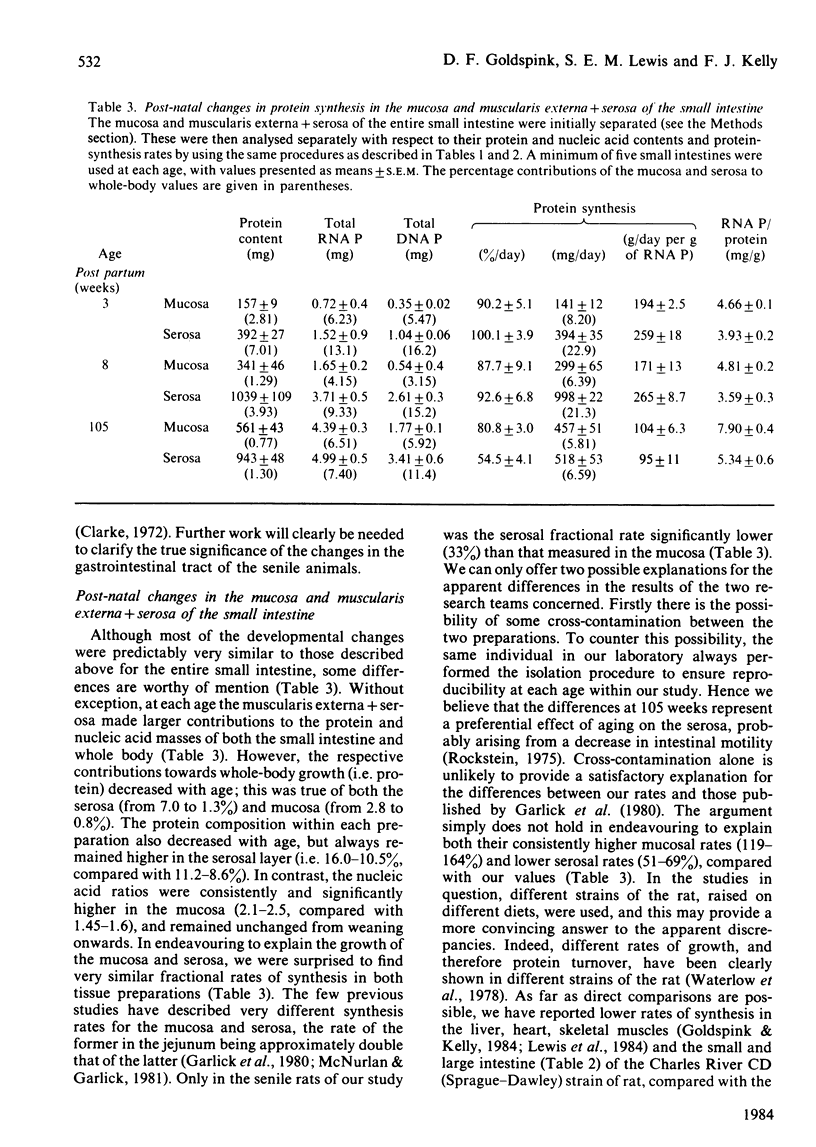
The developmental growth and associated changes in protein synthesis were measured (in vivo) in the combined small and large intestine from 18 days in utero to 105 weeks post partum. Similar post-natal (3-105 weeks) changes were also studied in the separated large and small intestine, and in the mucosal and muscularis externa + serosal layers of the small intestine. Although the protein and nucleic acid contents of the whole intestine increased throughout both pre- and post-natal life, the maximal (11%) intestinal contribution to whole-body growth occurred 3 weeks after birth; this value declined to only 2.5-3.5% at both extremes of the age range studied. Between the 18-day foetus and old age the fractional rate of protein synthesis decreased from 107 to 61% per day. This developmental decline (43%) was, however, much smaller than that found in most other body tissues over the same period. Similar developmental trends (between weaning and senility) were found in both the small and the large intestine when studied separately, the small intestine in all respects contributing proportionately more than the large intestine to both the combined intestinal and whole-body values. At each age the large intestine possessed significantly lower fractional rates of synthesis and associated ribosomal activities. For the most part, the fractional synthesis rates in the mucosa and serosa of the small intestine were very similar, with each declining slightly with increasing age. These developmental changes are discussed with respect to functional aspects within the gastrointestinal tract.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Kinzie J. L. Regulation of small intestinal protein metabolism. Gastroenterology. 1973 Mar;64(3):471–496. [PubMed] [Google Scholar]
- Alpers D. H. Protein synthesis in intestinal mucosa: the effect of route of administration of precursor amino acids. J Clin Invest. 1972 Jan;51(1):167–173. doi: 10.1172/JCI106788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. The effect of growth and of fasting on the number of villi and crypts in the small intestine of the albino rat. J Anat. 1972 May;112(Pt 1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Fern E. B., Garlick P. J. The specific radioactivity of the tissue free amino acid pool as a basis for measuring the rate of protein synthesis in the rat in vivo. Biochem J. 1974 Aug;142(2):413–419. doi: 10.1042/bj1420413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern E. B., Hider R. C., London D. R. Studies in vitro on free amino acid pools and protein synthesis in rat jejunum. Eur J Clin Invest. 1971 Jan;1(4):211–215. [PubMed] [Google Scholar]
- Forrester J. M. The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding. J Anat. 1972 Feb;111(Pt 2):283–291. [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Burk T. L., Swick R. W. Protein synthesis and RNA in tissues of the pig. Am J Physiol. 1976 Apr;230(4):1108–1112. doi: 10.1152/ajplegacy.1976.230.4.1108. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Kelly F. J. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J. 1984 Jan 15;217(2):507–516. doi: 10.1042/bj2170507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Kelly F. J., Goldspink D. F. Age-related growth of the spleen in normal and glucocorticoid treated rats. Comp Biochem Physiol A Comp Physiol. 1983;75(1):91–96. doi: 10.1016/0300-9629(83)90050-6. [DOI] [PubMed] [Google Scholar]
- Kelly F. J., Goldspink D. F. The differing responses of four muscle types to dexamethasone treatment in the rat. Biochem J. 1982 Oct 15;208(1):147–151. doi: 10.1042/bj2080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966 Dec 17;212(5068):1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P. CLASSIFICATION OF CELL POPULATIONS ON THE BASIS OF THEIR PROLIFERATIVE BEHAVIOR. Natl Cancer Inst Monogr. 1964 May;14:119–150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis S. E., Kelly F. J., Goldspink D. F. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J. 1984 Jan 15;217(2):517–526. doi: 10.1042/bj2170517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNurlan M. A., Garlick P. J. Contribution of rat liver and gastrointestinal tract to whole-body protein synthesis in the rat. Biochem J. 1980 Jan 15;186(1):381–383. doi: 10.1042/bj1860381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNurlan M. A., Garlick P. J. Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol. 1981 Sep;241(3):E238–E245. doi: 10.1152/ajpendo.1981.241.3.E238. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassner S. J., Li J. B. N tau-methylhistidine release: contributions of rat skeletal muscle, GI tract, and skin. Am J Physiol. 1982 Oct;243(4):E293–E297. doi: 10.1152/ajpendo.1982.243.4.E293. [DOI] [PubMed] [Google Scholar]


