Abstract
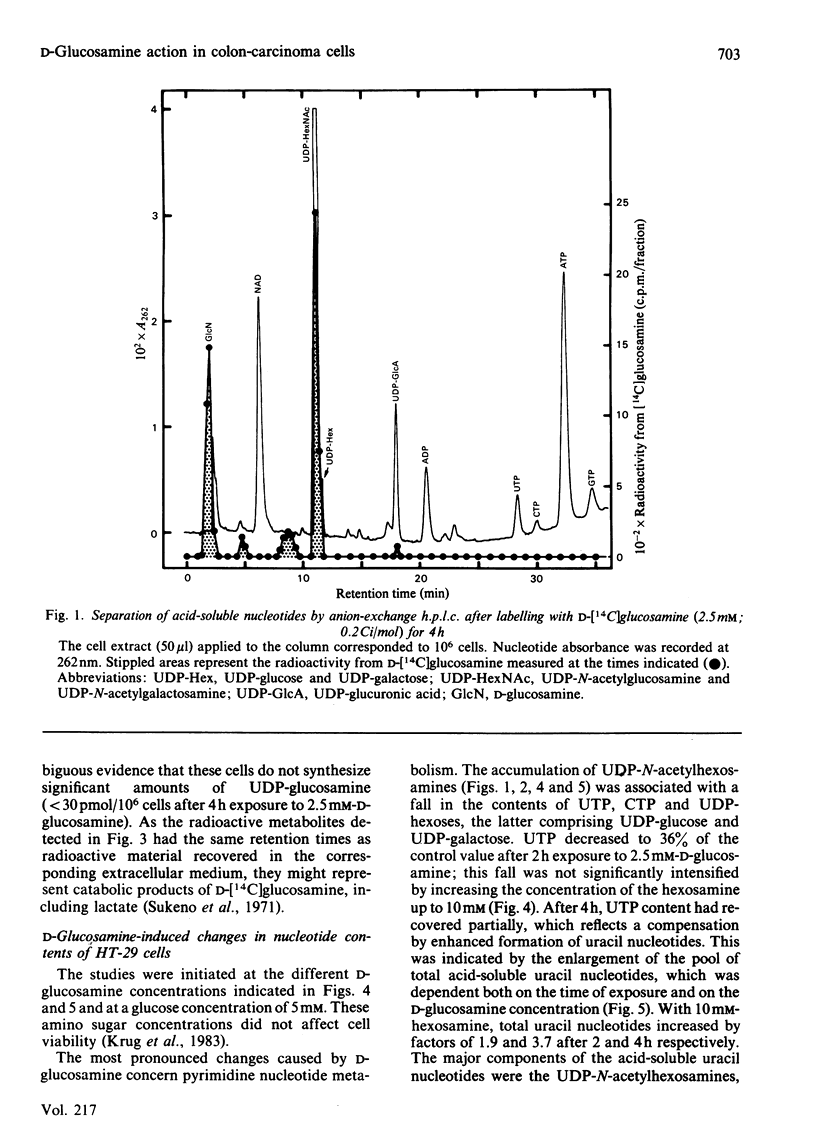
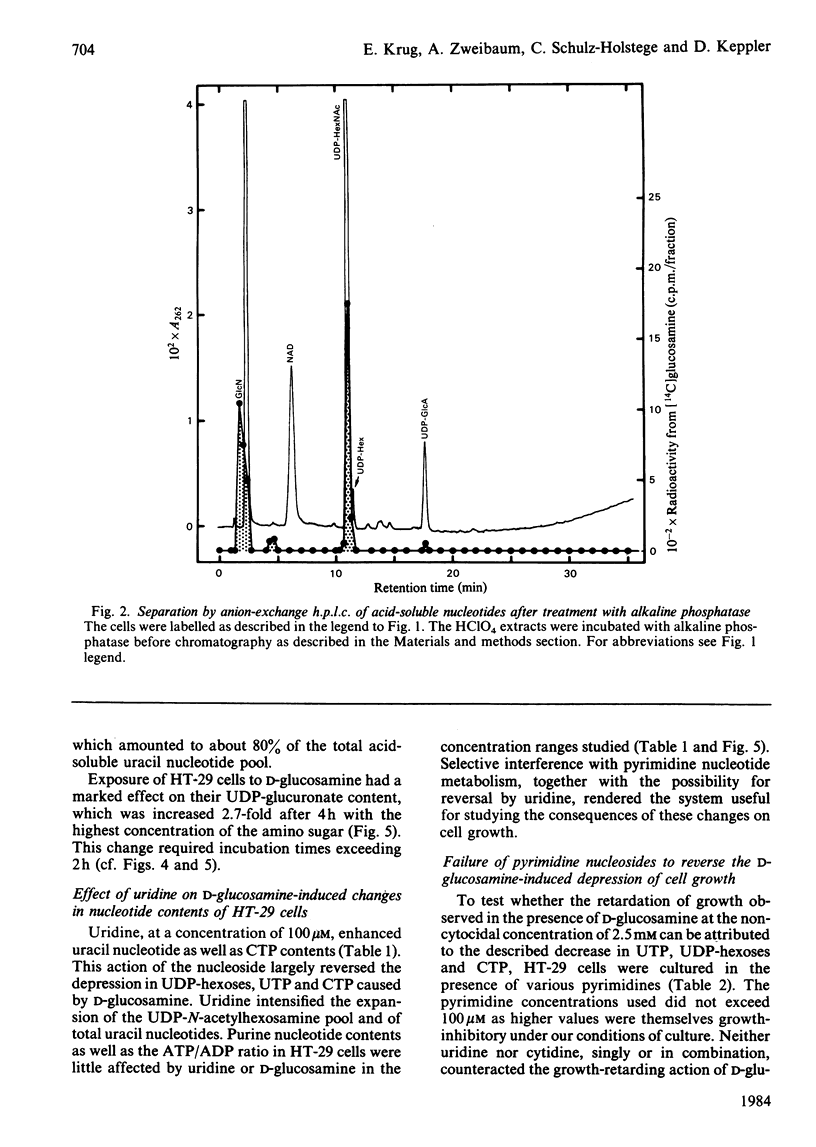
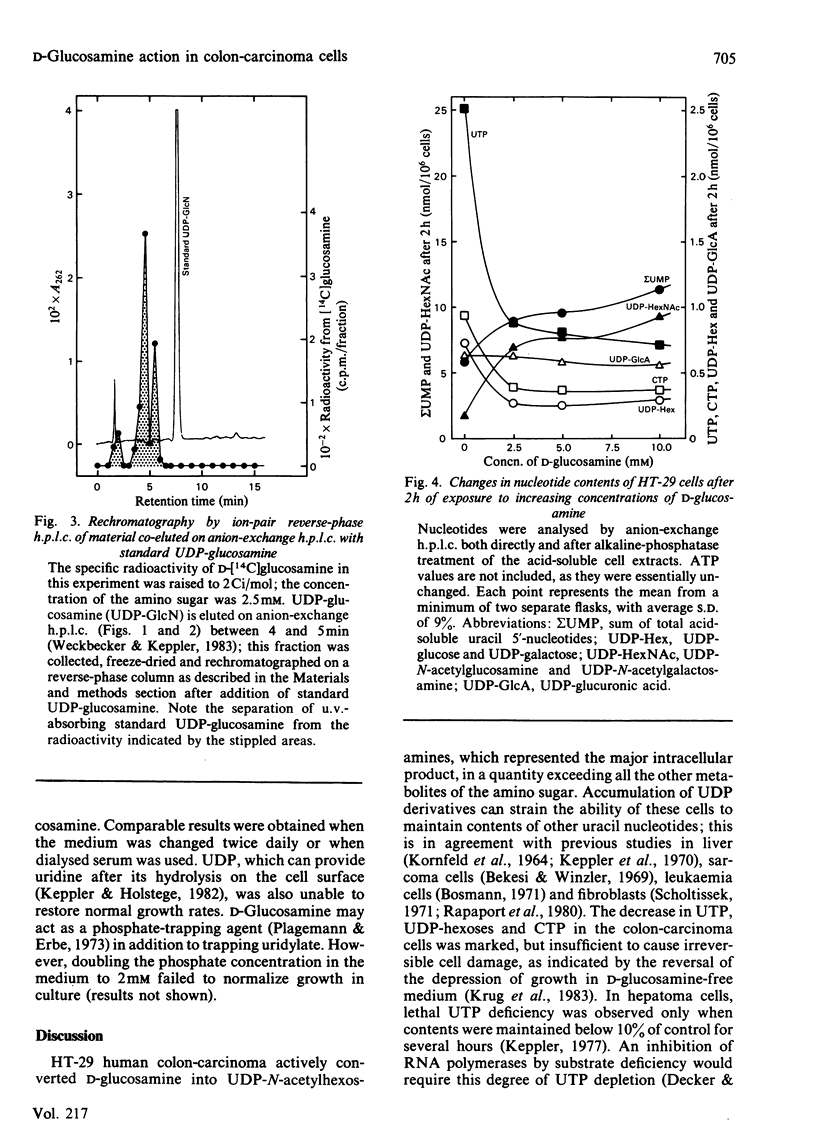
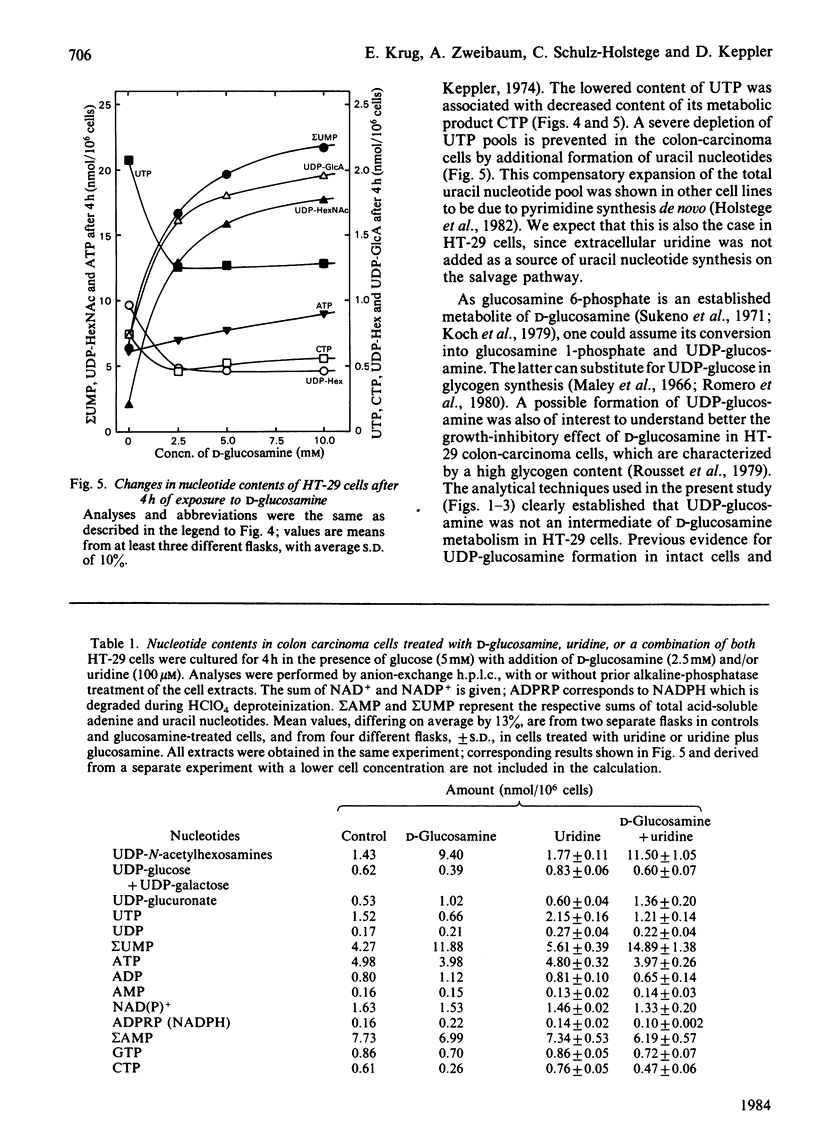
Human colon-carcinoma cells were exposed to D-glucosamine at 2.5, 5 and 10 mM, concentrations that were growth-inhibitory but not cytocidal in the presence of a physiological glucose concentration. Labelling of these HT-29 cells with D-[14C]-glucosamine, followed by nucleotide analyses, demonstrated that UDP-N-acetyl-hexosamines represented the major intracellular nucleotide pool and the predominant metabolite of the amino sugar. D-[14C]Glucosamine was not a precursor of UDP-glucosamine. After 4h exposure to D-glucosamine (2.5 mM), the pool of UDP-N-acetylhexosamines was increased more than 6-fold, whereas UTP and CTP were markedly decreased. UDP-glucuronate content increased by more than 2-fold, whereas purine nucleotide content was little altered. Uridine (0.1 mM) largely reversed the decrease in UTP, CTP, UDP-glucose and UDP-galactose, while intensifying the expansion of the UDP-N-acetylhexosamine pool. Uridine did not reverse the D-glucosamine-induced retardation of growth in culture. A 50% decrease in growth also persisted when uridine and cytidine, cytidine alone, or UDP, were added together with D-glucosamine. The growth-inhibitory effect of the amino sugar could therefore be best correlated with the quantitative change in the pattern of sugar nucleotides, and, in particular, with the many-fold increase in UDP-N-acetylglucosamine and UDP-N-acetylgalactosamine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekesi J. G., Winzler R. J. The effect of D-glucosamine on the adenine and uridine nucleotides of sarcoma 180 ascites tumor cells. J Biol Chem. 1969 Oct 25;244(20):5663–5668. [PubMed] [Google Scholar]
- Bosmann H. B. Inhibition of protein, glycoprotein, ribonucleic acid and deoxyribonucleic acid synthesis by D-glucosamine and other sugars in mouse leukemic cells L5178Y and selective inhibition in SV-3T3 compared with 3T3 cells. Biochim Biophys Acta. 1971 Jun 17;240(1):74–93. doi: 10.1016/0005-2787(71)90515-6. [DOI] [PubMed] [Google Scholar]
- Chelibonova-Lorer H., Gavazova E., Ivanov S., Antonova M. Effect of D-glucosamine on the content and synthesis of UDP-sugars and plasma membrane associated carbohydrates in chicken liver and hepatoma Mc-29. Int J Biochem. 1983;15(4):559–564. doi: 10.1016/0020-711x(83)90131-3. [DOI] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Interference with glycosylation of glycoproteins. Inhibition of formation of lipid-linked oligosaccharides in vivo. Biochem J. 1979 Oct 15;184(1):113–123. doi: 10.1042/bj1840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Skehan P. Membrane-active drugs potentiate the killing of tumor cells by D-glucosamine. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1172–1176. doi: 10.1073/pnas.77.2.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Liao J. C. Reversed phase high performance liquid chromatographic separations of nucleotides in the presence of solvophobic ions. Anal Chem. 1977 Dec;49(14):2231–2234. doi: 10.1021/ac50022a030. [DOI] [PubMed] [Google Scholar]
- Holstege A., Schulz-Holstege C., Henninger H., Reiffen K. A., Schneider F., Keppler D. O. Uridylate trapping induced by the C-2-modified D-glucose analogs glucosone, fluoroglucose, and glucosamine. Eur J Biochem. 1982 Jan;121(2):469–474. doi: 10.1111/j.1432-1033.1982.tb05811.x. [DOI] [PubMed] [Google Scholar]
- KORNFELD S., KORNFELD R., NEUFELD E. F., O'BRIEN P. J. THE FEEDBACK CONTROL OF SUGAR NUCLEOTIDE BIOSYNTHESIS IN LIVER. Proc Natl Acad Sci U S A. 1964 Aug;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Keppler D. O. Uridine triphosphate deficiency, growth inhibition, and death in ascites hepatoma cells induced by a combination of pyrimidine biosynthesis inhibition with uridylate trapping. Cancer Res. 1977 Mar;37(3):911–917. [PubMed] [Google Scholar]
- Koch H. U., Schwarz R. T., Scholtissek C. Glucosamine itself mediates reversible inhibition of protein glycosylation. A study of glucosamine metabolism at inhibitory concentrations in influenza-virus-infected cells. Eur J Biochem. 1979 Mar;94(2):515–522. doi: 10.1111/j.1432-1033.1979.tb12920.x. [DOI] [PubMed] [Google Scholar]
- Krug E., Dussaulx E., Rozen R., Griglio S., de Gasquet P., Zweibaum A. D-Glucosamine-induced increase of the glycerol-containing lipids in growing cultures of human malignant epithelial cells. J Natl Cancer Inst. 1983 Jan;70(1):57–61. [PubMed] [Google Scholar]
- Maley F., McGarrahan J. F., DelGiacco R. Galactosamine: a precursor of glycogen glucosamine. Biochem Biophys Res Commun. 1966 Apr 6;23(1):85–91. doi: 10.1016/0006-291x(66)90273-7. [DOI] [PubMed] [Google Scholar]
- Maley F., Tarentino A. L., McGarrahan J. F., Delgiacco R. The metabolism of d-galactosamine and N-acetyl-d-galactosamine in rat liver. Biochem J. 1968 May;107(5):637–644. doi: 10.1042/bj1070637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin M. J., Porter C. W., McKernan P., Bernacki R. J. The biochemical and ultrastructural effects of tunicamycin and D-glucosamine in L1210 leukemic cells. J Cell Physiol. 1983 Feb;114(2):162–172. doi: 10.1002/jcp.1041140204. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Transport and metabolism of glucosamine by cultured Novikoff rat hepatoma cells and effects on nucleotide pools. Cancer Res. 1973 Mar;33(3):482–492. [PubMed] [Google Scholar]
- QUASTEL J. H., CANTERO A. Inhibition of tumour growth by D-glucosamine. Nature. 1953 Feb 7;171(4345):252–254. doi: 10.1038/171252a0. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Christopher C. W., Ullrey D., Kalckar H. M. Selective high metabolic lability of uridine triphosphate in response to glucosamine feeding of untransformed and polyoma virus-transformed hamster fibroblasts. J Cell Physiol. 1980 Aug;104(2):253–259. doi: 10.1002/jcp.1041040216. [DOI] [PubMed] [Google Scholar]
- Rousset M., Chevalier G., Rousset J. P., Dussaulx E., Zweibaum A. Presence and cell growth-related variations of glycogen in human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1979 Feb;39(2 Pt 1):531–534. [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Sukeno T., Kikuchi H., Saeki H., Tsuiki S. Transformation of glucosamine to glycogen and lactate by ascites tumor cells. Biochim Biophys Acta. 1971 Jul 20;244(1):19–29. doi: 10.1016/0304-4165(71)90116-4. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Graff G., Moos M. C., Jr, Goldberg N. D. Separation of 5'-ribonucleoside monophosphates by ion-pair reverse-phase high-performance liquid chromatography. Anal Biochem. 1980 Sep 1;107(1):240–245. doi: 10.1016/0003-2697(80)90516-3. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Keppler D. O. Dual role of hexose-1-phosphate uridylyltransferase in galactosamine metabolism. Eur J Biochem. 1982 Nov;128(1):163–168. doi: 10.1111/j.1432-1033.1982.tb06947.x. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Keppler D. O. Separation and analysis of 4'-epimeric UDP-sugars by borate high-performance liquid chromatography. Anal Biochem. 1983 Jul 15;132(2):405–412. doi: 10.1016/0003-2697(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Studies on the control of hexosamine biosynthesis by glucosamine synthetase. Biochem J. 1971 Feb;121(4):711–720. doi: 10.1042/bj1210711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUSHOK W. D. Inhibition of glucolysis and fructolysis of Krebs 2 ascites carcinoma cells by chemical agents. Cancer Res. 1958 Sep;18(8 Pt 2):379–389. [PubMed] [Google Scholar]


