Abstract
Background
Patients with coronavirus disease 2019(COVID-2019) infections may still experience long-term effects, with fatigue being one of the most frequent ones. Clinical research on the long COVID in the Chinese population after infection is comparatively lacking.
Objective
To collect and analyze the long-term effects of non-severe COVID-19 infection patients and to develop a model for the prediction of fatigue symptoms.
Methods
223 non-severe COVID-19 patients admitted to one designated hospital were enrolled after finish all the self-designed clinical information registration form and nine-month follow-up. We explored the frequency and symptom types of long COVID. Correlation analysis was done on the neuropsychological scale results. After cluster analysis, lasoo regression and logistic regressions, a nomogram prediction model was produced as a result of investigating the risk factors for fatigue.
Results
A total of 108 (48.4%) of the 223 non-severe COVID-19 patients reported sequelae for more than 4 weeks, and of these, 35 (15.7%) had fatigue sequelae that were scale-confirmed. Other sequelae of more than 10% were brain fog (n = 37,16.6%), cough (n = 26,11.7%) and insomnia (n = 23,10.3%). A correlation between depression and fatigue was discovered following the completion of neuropsychological scale. The duration of hospitalization, the non-use of antiviral medications in treatment, IL-6 and CD16+CD56+ cell levels in blood are the main independent risk factors and predictors of fatigue sequelae in long COVID. Additionally, the neurology diseases and vaccination status may also influence the fatigue sequelae.
Conclusion
Nearly half of the patients infected with COVID-19 Omicron variant complained of sequelae, and fatigue was the most common symptom, which was correlated with depression. Significant predictors of fatigue sequelae included length of hospitalization, non-use of antiviral drug, and immune-related serum markers of IL-6 and CD16+CD56+ NK cell levels. The presence of neurology diseases and a lack of vaccination could also predict the occurrence of fatigue sequelae.
Keywords: SARS-CoV-2, COVID-19, Omicron, Long COVID syndrome, Fatigue
Highlights
-
•
This research represents the first prospective cohort study with long-term follow-up after the first Omicron wave in Shanghai, China.
-
•
This study thoroughly collected all pertinent data throughout the hospitalization period, promptly followed up, and included a series of neuropsychological scales to further quantify subjective symptoms.
-
•
The neurologists performed a face-to-face assessment of neurological manifestations, which ensured the reliability and professionalism of diagnosis.
1. Introduction
By now, the severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) had infected more than 700 million people worldwide. Although the virus's current virulence has significantly decreased, the effects of infection are receiving more attention, particularly after repeated infection with different waves of the virus. The potential effects on the world's social and medical environment, as well as the economic burden, have a significant impact on the human health (Machkovech et al., 2024), psychological state, and quality of life. Throughout the course of human evolution, we have also discovered that a wide range of bacteria and viruses can have a long-term impact on brain. For instance, the Epstein-Barr(EB) virus and the herpes virus may be associated with a number of neurodegenerative conditions, such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis (Marogianni et al., 2020; Marcocci et al., 2020; Zhang et al., 2022a). Studies on these neurodegenerative diseases have focused mostly on the long-term emergence of viral infection; as a result, there may be a variety of intervening factors, making it difficult to make a broad diagnosis based on pathophysiology at this time. As compared to other respiratory viruses, the novel coronavirus clearly has a high probability of causing neurological symptoms, and these symptoms are reliable and last longer than those caused by other virus infections (Hattab et al., 2024) Therefore, research on the long-term effects of the novel coronavirus on the nervous system may offer a chance for basic science and clinical data on the relationship between viral infection and degenerative diseases.
Long COVID, also known as postacute sequelae of SARS-CoV-2 infection(PASC), is receiving more and more attention in both clinical and basic research, especially in the present situation that SARS-CoV-2 is a threat to our daily lives (Thaweethai et al., 2023; Yong and Liu, 2022). The researcher estimate that infections persisting for at least 2 months might have occurred at a frequency of 1 in 200 to 1 in 1000 and even straight up to 50% (Machkovech et al., 2024; Fernández-de-las-Peñas et al., 2022). The emergence of sequelae has drawn attention even in the early stages of the epidemic. Studies conducted all over the world have demonstrated that when compared to other viral infections, long COVID occurs more frequently and with more severe symptoms (Sudre et al., 2021) that might even impair patients' ability to work due to long-term physical, social, and psychological harm. Such sequelae were initially thought to be primarily brought on by the early, high virulence of the virus and the greater harm to the entire body (Mohandas et al., 2023; Sherif et al., 2023). However, there hasn't been much clinical or basic research of the occurrence of this symptom.
Fatigue is the most common symptom of long COVID-19, and some studies have found that it can account for up to 40% (Baker et al., 2023; Joli et al., 2022). The majority of patients with long-term fatigue sequelae after infection were found to also have more neurophysiological abnormalities, inconsistent with previous hypothesis' assumptions that this symptom is only directly related to infection and immunity (Ortelli et al., 2021; Ceban et al., 2022). Consequently, taking into account the symptoms of fatigue may have a direct connection to the nervous system. Physical fatigue and mental fatigue are two different types of fatigue that are frequently present together in chronic fatigue. Fatigue is the most common sequelae, which has a significant impact on patients' quality of life (Saqib et al., 2024). As a result, obtaining and analyzing early clinical data can effectively predict and intervene the occurrence of symptoms. This study focuses on this common symptom in order to identify any clinical risk factors that might be responsible for this symptom's persistence.
2. Materials and methods
2.1. Patients
The study was designed as a prospective cohort study carried out in patients affected by COVID-19 during the Omicron wave in Shanghai back in 2022. All patients which were confirmed as positive results for SARS-CoV-2 on real-time RT-PCR were hospitalized in a designated hospital. Being over 18 years old, conscious, cognitively and mentally intact, and linguistically capable of responding to the anamnestic interview at discharge and follow-up were inclusion criteria. Drop outs happened when the patients needed to be transferred to ICU, loss to follow-up or re-infected in 6 months.
2.2. Data collection
The medical records and extracted data on general characteristics, laboratory tests, chest computed tomography(CT) reports, treatment, prognosis, RT-PCR dynamic detection of SARS-CoV-2, treatment and prognosis during hospitalization were recorded according to our previous study (Shen et al., 2023). All symptoms were mainly subjectively expressed by patients. All anamnestic interviews were collected by clinicians from the general wards where patients were hospitalized, and reviewed and confirmed by two trained neurologists.
Chest CT scans were assessed in all of the patients. The clinical severity of COVID-19 was classified by reports of chest CT according to the latest version of the guidelines (Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 9th Version), 2022). The specific lesions described in the CT report were also classified and summarized.
Blood samples were also collected on admission. Routine blood biochemistry, coagulation function and myocardial enzymes were collected and analyzed according to our previous study (Shen et al., 2023). In additional, inflammatory cytokines (including Interleukin-6[IL-6] and Tumor necrosis factor-ɑ[TNF-ɑ]) and lymphocyte subsets cluster of differentiation (CD) (CD3+, CD3+CD4+, CD3+CD8+, CD16+CD56+, CD19+) were also collected.
Nine month after discharge, patients received a follow-up from the clinical staff and finished Fatigue Scale-14 (FS-14), Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder (GAD-7). Long COVID is defined as the continuation of symptoms for longer than 4 weeks after infection. FS-14 scores greater than 6 indicate objective fatigue symptoms. PHQ-9 score of 10 or higher indicates depressive symptom. GAD score of 10 or higher indicates anxious symptom (Morriss et al., 1998; xin et al., 2020).
2.3. Statistical analysis
Statistical Package for the Social Sciences for Windows (SPSS version 26.0; IBM Corp, Armonk, NY, USA) and R software (version 4.3.1, http://www.r-project.org)was used to perform the statistical analyses. Categorical data was expressed as absolute frequencies and percentages where appropriate. Continuous variables were expressed as medians (interquartile ranges [IQR], Q1-Q3). Categorical data was compared using Pearson's chi-square test or Fisher's exact test. Mann-Whitney U test and logistic regression were used to compare the differences for continuous variables between groups. Collinearity indicators were screened out by unsupervised cluster analysis and lasso regression. The final variables obtained by lasso regression were subsequently used to binary logistic regressions and build nomogram prediction model. The discriminatory ability of the nomogram was validated by using receiver operating characteristics (ROC) curves. The process was conducted 1000 times at random. The area under the curve (AUC) of the 1000 verifications was calculated with the R package. Odds ratios and 95% confidence intervals were also estimated for every single factor. A p-value (two-sided) less than 0.05 was considered significant.
3. Results
The study screened a total of 351 patients who underwent various tests and face-to-face interviews during hospitalization. 87 patients were lost to follow-up due to a change in contact information, 26 patients were lost to follow-up due to refusal, and 3 patients had passed away as of the most recent follow-up. Lastly, 223 cases were enrolled in the study of fatigue of long COVID.
3.1. Clinical symptoms in Long-COVID
Among the 223 patients, 108 (48.4%) self-complained of sequelae for more than 4 weeks. The longest case among the 223 patients lasted for 7 months, and the majority of cases vanished in 3–4 months. In the second COVID-19 pandemic wave from December 2022 to January 2023, 27 cases (12.1%) were confirmed to have been re-infected with COVID-19, and 2 cases manifested symptoms but underwent no diagnostic testing.
Fatigue (n = 51, 22.8%) was the most common self-reported symptom in long COVID, while brain fog (n = 5137, 16.6%), followed by cough (n = 26,11.7%), and insomnia (n = 23,10.3%). See Fig. S1 for other symptoms. It is worth noting that in patients with self-reported fatigue, after completing the FS-14 scale, only 35(68.6%) were ultimately considered to have definite fatigue symptoms.
After completing the PHQ-9 and GAD-7, patients who had emotional disorders revealed that 5 of them had both anxiety and depressive symptoms, 2 had simple anxietic symptom, and 9 had simple depressive symptom. After grouping the two emotional disorders according to fatigue, it was found that, the number of depressed patients with fatigue sequelae increased significantly (31.4% vs 1.6%, p < 0.001). However, there was no statistical significance between anxiety state and fatigue sequelae (see Table 1).
Table 1.
Clinical Scale Outcome of Long COVID patient with fatigue.
| Variables | All | With Fatigue | Without Fatigue | P value |
|---|---|---|---|---|
| Total Score | 2.54 ± 3.47 | 9.34 ± 2.00 | 1.27 ± 1.82 | <0.001∗∗∗ |
| Physical Fatigue Score | 1.61 ± 2.44 | 6.14 ± 1.75 | 0.77 ± 1.38 | <0.001∗∗∗ |
| Mental Fatigue Score | 0.94 ± 1.52 | 3.20 ± 1.84 | 0.52 ± 1.00 | <0.001∗∗∗ |
| Depression(PHQ-9)(0–27) | 0.98 ± 3.07 | 6.89 ± 4.92 | 0.77 ± 2.56 | <0.001∗∗∗ |
| Yes (≥10) | 14(6.3) | 11(31.4) | 3(1.6) | |
| Anxiety(GAD-7)(0–21) | 1.73 ± 3.76 | 3.26 ± 4.77 | 0.56 ± 2.43 | 0.750 |
| Yes (≥10) | 7(3.1) | 3(8.6) | 4(2.1) |
3.2. Demographic, clinical characteristics and laboratory findings
Among 223 patients with confirmed SARS-CoV2 infection, 35 cases (15.7%) were classified into the fatigue sequelae group after completing the scale. Baseline data and demographic characteristics are shown in Table 2. After univariate analyses, the proportion of fatigue patients with chronic liver disease was higher (11.4%). Although there was no clear statistical significance in the univariate analysis, the results showed that patients with underlying neurological diseases, especially Parkinson's disease, had a higher probability of fatigue sequelae. In clinical treatment, patients who received early glucocorticoid therapy had a higher percentage of fatigue sequelae (5.9% vs 17.1%), whereas patients who received early Paxlovid had a lower percentage (64.9% vs 48.6%). The likelihood of fatigue sequelae increases with length of hospital stay.
Table 2.
Demographic and clinical characteristic.
|
n(%) or median[IQR] |
p- value | |||
|---|---|---|---|---|
| Total |
With fatigue |
Without fatigue |
||
| (n = 223) | (n = 35) | (n = 188) | ||
| Characteristics | ||||
| Age, y | 71 (62, 81) | 67 (61, 82) | 71 (62, 80) | 0.124 |
| Gender | ||||
| Female | 120 (53.8) | 22 (62.9) | 98 (52.1) | 0.720 |
| Male | 103 (46.2) | 13 (37.1) | 90 (47.9) | |
| BMI | 23.1(21.0,25.4) | 23.9(21.4,26.3) | 23.1(20.9,25.3) | 0.382 |
| Comorbidities | ||||
| Any | 180 (80.7) | 31 (88.6) | 149 (79.3) | 0.249 |
| Hypertension | 121 (54.3) | 19 (54.3) | 102 (54.3) | 0.680 |
| Diabetes | 48 (21.5) | 6 (17.1) | 42 (22.3) | 0.376 |
| Cardiac disease | 69 (30.9) | 14 (40.0) | 55 (29.3) | 0.256 |
| Neurology disease | 64 (28.7) | 14 (40.0) | 50 (26.6) | 0.093 |
| Cerebrovascular disease | 44 (19.7) | 9 (25.7) | 35 (18.6) | 0.295 |
| Dementia | 13 (5.8) | 2 (5.7) | 11 (5.9) | 0.926 |
| Parkinson disease | 11 (4.9) | 4 (11.4) | 7 (3.7) | 0.066 |
| Emotional disorder | 7 (3.1) | 2 (5.7) | 5 (2.7) | 0.353 |
| Epilepsy | 3 (1.3) | 1 (2.9) | 2 (1.1) | 0.417 |
| Chronic lung disease | 33 (14.8) | 5 (14.3) | 28 (14.9) | 0.699 |
| Chronic liver disease | 10 (4.5) | 4 (11.4) | 6 (3.2) | 0.043∗ |
| Chronic kidney disease | 6 (2.7) | 2 (5.7) | 4 (2.1) | 0.248 |
| Cancer | 11 (4.9) | 3 (8.6) | 8 (4.3) | 0.289 |
| Comorbidities≥2 | 130 (58.3) | 22 (62.9) | 108 (57.4) | 0.483 |
| Vaccinated | 95 (42.6) | 11 (31.4) | 84 (44.7) | 0.271 |
| Booster injection | 53 (23.8) | 4 (11.4) | 49 (26.1) | 0.587 |
| Degree of severity | ||||
| Mild | 178 (79.8) | 29 (82.9) | 149 (79.3) | 0.796 |
| Moderate | 45 (20.2) | 6 (17.1) | 39 (20.7) | |
| Treatment | ||||
| Traditional Chinese Medicine | 211 (94.6) | 34 (97.1) | 177 (94.1) | 0.479 |
| Antibiotics | 50 (22.4) | 12 (34.3) | 38 (20.2) | 0.058 |
| Anticoagulation | 31 (13.9) | 6 (17.1) | 25 (13.3) | 0.496 |
| Glucocorticoid | 17 (7.6) | 6 (17.1) | 11 (5.9) | 0.019∗ |
| Thymosin | 43 (19.3) | 8 (22.9) | 35 (18.6) | 0.560 |
| Paxlovid | 139 (62.3) | 17 (48.6) | 122 (64.9) | 0.037∗ |
| Nutritional support | 62 (27.8) | 14 (40.0) | 48 (25.5) | 0.224 |
| Clinical outcomes | ||||
| Length of hospitalization, d | 9 (5, 11) | 10 (9, 13) | 8.5 (5, 11) | 0.002∗∗ |
Univariate regression revealed that the group with fatigue sequelae had significantly higher serum IL-6 levels (p = 0.048). The ratio of lymphocyte to monocyte and CD16+CD56+ NK cells were different between the two groups but showed no statistical significance. Other tests showed no significant difference between the two groups. There was no significant difference between the two groups in chest CT imaging results. Please see Table 3 for more information.
Table 3.
Laboratory findings.
| Items |
With fatigue |
Without fatigue |
|
|---|---|---|---|
| (n = 35) |
(n = 188) |
p-value |
|
| Blood test, median[IQR] | |||
| WBC count, ∗10ˆ9/L | 5.5 (3.7, 6.2) | 5.2 (3.9, 6.3) | 0.749 |
| Neutrophil/Lymphocyte, | 0.4 (0.3, 0.7) | 0.4 (0.3, 0.5) | 0.072 |
| CD3+, % | 780.0 (581.0, 1051.0) | 746.0 (589.3, 958.0) | 0.470 |
| CD3+CD4+, % | 472.0 (297.0, 705.0) | 448.0 (334.0, 548.0) | 0.479 |
| CD3+CD8+, % | 265.0 (165.0, 343.0) | 253.0 (170.0, 356.0) | 0.623 |
| CD16+CD56+, % | 194.0 (134.0, 248.0) | 242.0 (179.0, 373.0) | 0.075 |
| CD19+, % | 129.0 (93.0, 190.0) | 134.0 (85.0, 208.0) | 0.306 |
| Hemoglobin,g/L | 128.0(121.5136.5) | 131.0(120.8144.0) | 0.355 |
| Platelet,∗10ˆ9/L | 193.0 (154.5, 224.5) | 179.5 (145.8, 227.0) | 0.723 |
| C-reactive protein, mg/L | 3.7 (1.8, 7.7) | 3.7 (1.6, 9.0) | 0.723 |
| Creatine kinase, U/L | 79.0 (60.5, 112.0) | 85.5 (58.0, 129.8) | 0.556 |
| Lactate dehydrogenase, U/L | 200.0 (173.0, 229.0) | 208.0 (181.0, 236.5) | 0.699 |
| ALT, U/L | 17.0 (13.0, 36.0) | 17.0 (12.0, 26.0) | 0.688 |
| AST, U/L | 26.0 (21.5, 38.0) | 25.0 (19.5, 33.0) | 0.720 |
| TBil, mmol/L | 10.2 (8.3, 12.8) | 10.4 (7.8, 14.2) | 0.546 |
| Albumin, g/L | 39.0 (38.0, 43.5) | 40.0 (37.8, 43.0) | 0.903 |
| D-dimer, mg/L | 0.4 (0.2, 0.6) | 0.4 (0.2, 0.7) | 0.826 |
| Blood urea nitrogen, | 4.7 (4.1, 5.6) | 5.1 (4.2, 6.3) | 0.292 |
| Creatinine, umol/L | 70.0 (59.5, 83.5) | 76.0 (63.0, 88.5) | 0.144 |
| IL-6, pg/mL | 7.3 (4.8, 9.2) | 5.1 (3.5, 7.4) | 0.048∗ |
| IL-8, pg/mL | 11.7 (8.5, 22.1) | 12.7 (9.0, 18.0) | 0.570 |
| TNF-α, pg/mL | 9.4 (7.3, 11.5) | 8.7 (6.5, 11.5) | 0.891 |
| Lactic acid, mmol/L | 1.4 (0.9, 2.1) | 1.3 (1.0, 1.7) | 0.696 |
| ESR, mm/h | 17.5 (8.0, 26.8) | 18.0 (11.0, 28.0) | 0.744 |
| Myohemoglobin, μg/L | 26.9 (19.6, 40.9) | 30.3 (20.6, 49.9) | 0.873 |
| PT, s | 10.9 (10.5, 11.3) | 10.9 (10.5, 11.5) | 0.310 |
| APTT, s | 27.6 (26.1, 29.3) | 28.5 (26.7, 30.7) | 0.114 |
| Fibrinogen, g/L | 2.9 (2.5, 3.5) | 3.1 (2.7, 3.6) | 0.341 |
| Chest CT findings, n(%) | |||
| Bilateral lung involvement | 18 (51.4) | 75 (39.9) | 0.281 |
| Patchy shadowing | 1 (2.9) | 9 (4.8) | 0.700 |
| Ground-glass opacity | 3 (8.6) | 7 (3.7) | 0.216 |
| Interstitial abnormalities | 3 (8.6) | 14 (7.4) | 0.819 |
| Consolidation | 2 (5.7) | 2 (1.1) | 0.089 |
3.3. Predictive nomogram for fatigue sequelae
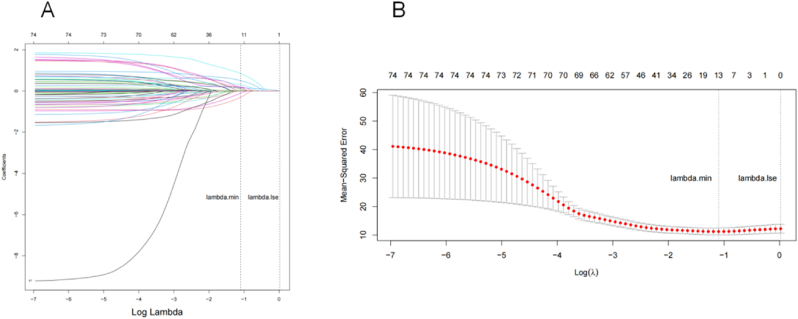
A total of 106 different types of clinical features were collected and 6 variables with data integrity below 35% were excluded from the analysis.100 variables were chosen for unsupervised cluster analysis (see details for features selection in Fig. S1). Then, after LASOO regression analysis (Fig. 1), 13 clinical features were chosen for analysis, including vaccination status, neurology disease, length of hospitalization, glucocorticoid, Paxlovid application, fever, cough, lymphocyte monocyte ratio, CD16+CD56+, IL-6, and APTT.
Fig. 1.
LASSO regression algorithm.
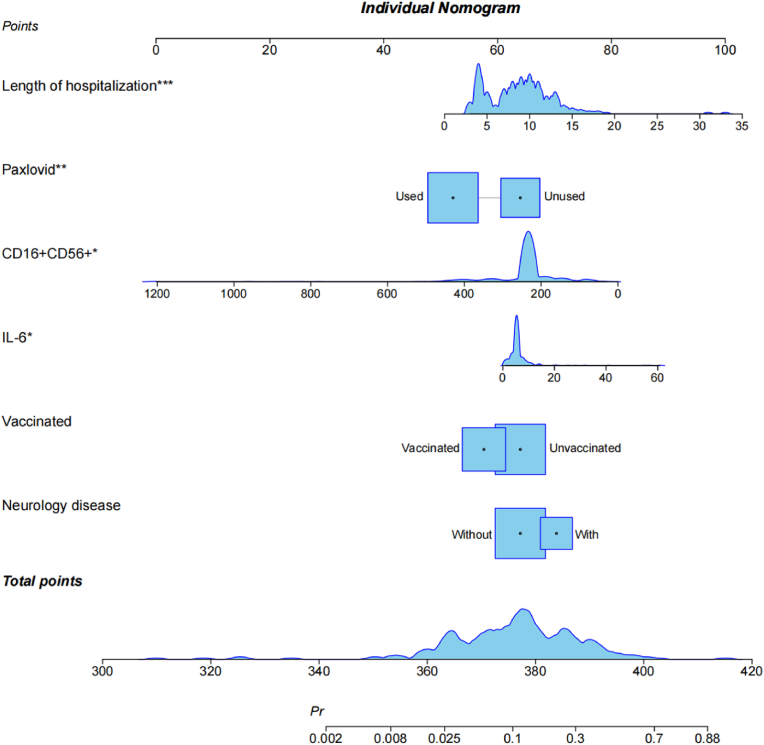
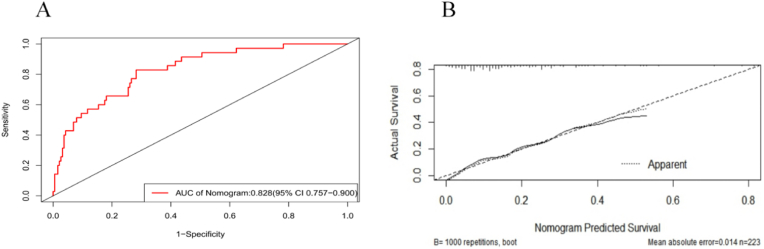
The individual scores of each predictor (0–100) are added to create the total score that is used to build the nomogram prediction model. Applied logistic regression after including each of the 13 factors in the preorder screening using R software. Then, a nomogram prediction model was established using the bootstrap method (1000 repeated sampling times). Variables with test coefficient points greater than 0.1 that had little bearing on the prediction were removed from the final analysis. Four of the six predictors that underwent the Logistic Regression Test and were eliminated demonstrated statistical differences (see Table S1). Fig. 2 displays the final findings. Fig. 3 displays the ROC curve that the model's internal validation C value was 0.828 (95% CI: 0.757–0.900) and correction curve. ROC and AUC for every single factor were displayed in Fig. S3 and Table S2.
Fig. 2.
Nomogram prediction model for fatigue in Long COVID.
Fig. 3.
ROC and AUC of nomogram prediction model.
4. Discussion
The sequelae have been discovered in more people, though, as the virus strain has changed and its virulence has decreased. It was reported that nearly 30% of COVID-19 patients may experience sequelae, with fatigue symptom ranking among the top three in a summary of various clinical data (Thaweethai et al., 2023; Al-Aly et al., 2024). These sequelae have a negative impact on many people's ability to work and normal lives after infection. A total of 223 patients finally completed the follow-up, and the results showed that: (1) Nearly 50% of the patients reported having long COVID syndrome, and the most common symptom was fatigue; (2) About 22.9% of them had subjective fatigue feelings. Following completion of the scale, 15.7% of them still manifested objective fatigue; (3) Compared with other emotional disorders, depression is more likely to occur in patients with fatigue sequelae; (4) Extensive early hospitalization and non-use of antiviral medications may be independent risk factors for the persistence of fatigue symptoms; (5) The persistence of fatigue sequelae may also be related to the IL-6 and CD16+CD56+ NK cell levels in the blood; (6) Neurology disease and a lack of vaccination and are potential predicators of fatigue as well.
First of all, the findings of our study demonstrate that 10% of people who contracted COVID-19 again after being exposed to the second pandemic wave, which is consistent with the findings of many other studies. This finding suggests that the study population was chosen in accordance with the epidemiological characteristics of earlier research. Our study showed that more than 20% of patients reported fatigue symptoms. But in fact, after being evaluated by FS-14 scale, the final incidence of fatigue symptoms is about 15%, which is consistent with the results of previous reviews reported around the world (Thaweethai et al., 2023; Frontera et al., 2022). In actuality, the symptoms of fatigue are very challenging to define and distinguish form subjective symptom such as brain fog and depression (Theoharides et al., 2021).Unfortunately brain fog lacks standardized clinical criteria despite being frequently mentioned both in COVID-19 and long COVID. Considerable number of brain fog studies that could be quantified were not applicable to telephone follow-up according to the review (Leaky blood, 2024). However, in some other studie (Van Der Feltz-Cornelis et al., 2024; Zhao et al., 2022; Aghajani Mir), it was difficult to distinguish between brain fog and mental fatigue and they were finally grouped into the same category of outcome variables. But there was no collinearity between fatigue and brain fog in the sequelae symptoms in our study, indicating that the they might not be completely related. Therefore, it is more objective to choose the fatigue symptoms quantified by the scale as the research object in our research. The fatigue symptoms themselves have long been thought to be related to the inflammatory state (Townsend et al., 2020). Studies have also speculated that this symptom may be connected to neuropsychological conditions, the most likely cause of which may be related to modifications in cortical microstructure and a decline in GABA function (Ortelli et al., 2022; Manganotti et al., 2023). More information is needed at this time.
The correlation scale used in this study revealed that there is some correlation between fatigue and depression. This has also been brought up in other sequela studies. However, because most studies lacked baseline data and neuropsychological scale support, no pertinent clinical studies on big data have yet been suggested to investigate this phenomenon. However, because depression is a negative emotional disorder that can easily result in fatigue, loss of interest, loss of appetite, insomnia, and other symptoms, there is some overlap between the two symptoms that makes it challenging to diagnose clinically and impossible for a neuropsychological scale to tell them apart. Studies have also revealed that the SARS-CoV-2 may alter related brain structures like the cerebral cortex and hippocampus abnormally (Klein et al., 2021). These abnormal changes may then affect related neurotransmitters and neural circuits, resulting in subjective symptoms. To ascertain whether there is a connection between the occurrence of neurodegenerative diseases brought on by external factors, long-term clinical follow-up certification and basic research support are still required.
The duration of the hospital stay was the indicator with the highest correlation among the early predictive risk factors affecting the occurrence of fatigue sequelae. The likelihood of fatigue sequelae increases with the amount of time the virus remains in the body (Leng et al., 2023). This outcome is unquestionably in line with clinical and public perception. The virus in the body not only affects the respiratory system but also spreads to various organs, resulting in abnormal organ function. ACE2 receptors are abundant in blood vessels, the liver, kidneys, and other organs. When viruses attack these organs, they may result in specific microvascular and microstructural lesions (Ceban et al., 2022; Sandler et al., 2021), which ultimately cause fatigue. However, it's also possible that the aforementioned modifications in the brain's neurotransmitters, microcirculation, and brain microstructure are the result of modifications (Baker et al., 2023; Mazurkiewicz et al., 2023). Whatever the reason, a persistently high virus titer in the body harm human organs more and encourages the emergence and persistence of sequelae.
Nirmatrelvir-ritonavir (trade name: Paxlovid) is an effective antiviral drug developed by Pfizer for the SARS-CoV-2 (Marzolini et al., 2022). The medication is a conforming formulation in which nematavir, an effective inhibitor of viral major protease or 3-chymotrypsin-like protease, is combined with ritonavir, an inhibitor of HIV-1 protease and CYP3A, to increase blood concentration. RNA viruses (Reina and Iglesias, 2022), such as human immunodeficiency viruses(HIV), have naturally high mutation rates. As a result, these viruses are easily able to evade selective drug targets and develop resistance through mutations in specific amino acid residues within a single therapeutic environment. In clinical settings, this drug can successfully prevent viral replication and lower the virus's titer in the body. In numerous clinical studies, it was discovered that this medication can successfully prevent the development of sequelae in a variety of long-term symptoms in addition to encouraging nucleic acid to turn negative early (Wang et al., 2023). Nevertheless, some pertinent studies have noted that the drug's side effects may hasten cartilage aging and degeneration by triggering endoplasmic reticulum stress and interfering with REDOX homeostasis, leading to associated neuromuscular complications (Kong et al., 2022). However, our research revealed no glaring drug-related consequences. As a result, our study also supported the idea that nematavir/ritonavir can effectively lessen fatigue sequelae when used early.
CD16+CD56+ NK cell level is a specific cellular molecular marker on the surface of immune cells, and is another marker of immune response in addition to cytokines. CD16+CD56+ generally refers to the count of natural killer cells (NK) in peripheral blood. In the inflammatory form of COVID-19 and secondary hemocytocytophagotic lymphohistiocytoid syndrome, the inflammatory microenvironment may change the balance and lessen the effector function of NK cells (Osman et al., 2020), particularly in the case of systemic cell microenvironment ischemia brought on by the acute phase of infection, further decreasing NK cell cytotoxicity. NK cells also express ACE2, which has the ability to encourage NK cell apoptosis and reduce its cytotoxicity after infection (Zhang et al., 2022b; Witkowski et al., 2021). Patients with severe disease have lower total NK cell counts than those with mild disease in those with COVID-19 infection, which is correlated with the severity of symptoms (Leng et al., 2023).
A number of systemic symptoms associated with COVID-19 infection are thought to be caused by cytokine storms and autoimmune factors (Rabaan et al., 2021). In many studies, the excretion of IL-6 has been regarded as one of the major cytokines. IIL-6 is a cytokine with multiple functions that affects hematopoiesis, immunity, and inflammation in addition to being essential for the body's response to injury and infection. IL-6 plays a crucial role in the normal growth and operation of T lymphocytes and B lymphocytes, particularly in terms of immune effects, and it is linked to the occurrence of chronic inflammation. According to pertinent studies, IL-6 inhibitors can be used in the early stages of severe COVID-19 infections to reduce immune damage brought on by inflammatory storms. Since serum IL-6 has been linked to atypical neurological symptoms like olfactory disorders and brain fog (Kappelmann et al., 2021; Schultheiβ et al., 2022), it has also been considered one of the indicators of systemic immune symptoms following COVID-19 infection. According to our research, it can be a predictor of fatigue sequelae in long COVID.
By combining the two sets of data, it is possible to assess the SARS-CoV-2's effect on autoimmunity in greater detail, which suggests that fatigue is strongly associated with autoimmunity. Multiple studies have confirmed that it may be connected to the overactivation of autoimmune and chronic inflammation in the brain and throughout the body (Varchetta et al., 2021). Therefore, at the early stages of infection, blood IL-6 and CD16+CD56+ NK cells can reflect the level of systemic immune response, and a strong autoimmune response may be the most important pathophysiological basis of long COVID.
The occurrence of fatigue sequelae is also correlated with chronic neurology diseases and vaccination status. In the univariate analysis, neurological conditions, particularly Parkinson's syndrome, have a higher probability of causing fatigue sequelae. Numerous clinical studies have made reference to this idea (Thaweethai et al., 2023), which may be connected to the deterioration of brain structure and function brought on by underlying neurological diseases, which exacerbates the feeling of fatigue. The univariate analysis did not reveal the vaccination status to be a particularly significant difference, which may be influenced by a number of variables including age and comorbidities. The presence of fatigue sequelae can still be predicted by vaccination status even when using more sophisticated machine algorithms like lasoo regression. The recombinant novel coronavirus vaccine, which uses adenovirus as the carrier, is currently primarily administered in China. Its protection depends more on the number of vaccinations received, but the side effects are minimal. Through vaccination, the body's viral load can be reduced, but it may also lessen the inflammatory storm caused by the virus's immune response (Akova and Unal, 2021; Belayachi et al., 2022). This not only lessens the clinical symptoms of the initial infection but also significantly lowers the subsequent immune response.
Our study is a prospective cohort investigation of fatigue sequelae in a Chinese population after infection with COVID-19 Omicron variants. This study, in contrast to earlier ones, thoroughly collected all pertinent data throughout the hospitalization period, promptly followed up, and included a series of neuropsychological scales to further quantify subjective symptoms. However, there are some limitations to this study. This is one single-center study with limited sample size. The high loss rate during the follow-up period further reduces the sample size, which raises the possibility of skewed results. The baseline neuropsychological scale cannot be completed due to the potential effects of the unique social environment. The patients were at the peak of the pandemic, so it was difficult to collect additional neuroimaging information. All of the neuropsychological scales used in this study were completed over the phone, and because of potential communication and expression barriers, some results may not be accurately understood or expressed.
5. Conclusions
This study is a prospective cohort study with long-term follow-up that focuses on fatigue sequelae, the most prevalent symptom in long COVID-19. It also includes serial neuropsychological scales to further quantify and objectively assess the findings and creates a prediction model using a machine learning calculation method. The results showed that nearly half of the patients may have long-term COVID-19 sequelae, and the incidence of fatigue symptoms is greater than 10%. The duration of hospitalization, the non-use of antiviral medications early in treatment, and the blood levels of IL-16 and CD16+CD56+ are the main independent risk factors and predictors that can influence the occurrence of fatigue. Additionally, the presence of neurology diseases and a lack of vaccination are important predictors of the development of fatigue sequelae. Additional basic research is required to confirm the pertinent results, particularly the contributors of immune factors.
CRediT authorship contribution statement
Xiao-Lei Shen: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Investigation, Data curation, Conceptualization. Yu-Han Jiang: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Shen-Jie Li: Investigation, Data curation, Conceptualization. Xin-Yi Xie: Investigation, Data curation. Yu Cheng: Investigation, Formal analysis, Data curation. Li Wu: Software, Resources, Methodology. Jun Shen: Visualization, Validation, Resources, Conceptualization. Wei Chen: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Conceptualization. Jian-Ren Liu: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100889.
Contributor Information
Wei Chen, Email: david_chen8106@hotmail.com.
Jian-Ren Liu, Email: liujr021@sjtu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
The authors do not have permission to share data.
References
- Aghajani Mir M. Brain fog: a narrative review of the most common mysterious cognitive disorder in COVID-19. Mol. Neurobiol. Published online October 24, 2023. doi:10.1007/s12035-023-03715-y. [DOI] [PubMed]
- Akova M., Unal S. A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):276. doi: 10.1186/s13063-021-05180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z., Davis H., McCorkell L., et al. Long COVID science, research and policy. Nat Med. 2024;30(8):2148–2164. doi: 10.1038/s41591-024-03173-6. [DOI] [PubMed] [Google Scholar]
- Baker A.M.E., Maffitt N.J., Del Vecchio A., et al. Neural dysregulation in post-COVID fatigue. Brain Communications. 2023;5(3) doi: 10.1093/braincomms/fcad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayachi J., Obtel M., Mhayi A., Razine R., Abouqal R. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco. Lau E.H.Y., editor. PLoS One. 2022;17(12) doi: 10.1371/journal.pone.0278546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 9th Version) Infectious Diseases & Immunity. 2022;2(3):135–144. doi: 10.1097/ID9.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-las-Peñas C., Notarte K.I., Peligro P.J., et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14(12):2629. doi: 10.3390/v14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera J.A., Yang D., Medicherla C., et al. Trajectories of neurologic recovery 12 Months after hospitalization for COVID-19: a prospective longitudinal study. Neurology. 2022;99(1):e33–e45. doi: 10.1212/WNL.0000000000200356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattab D., Amer M.F.A., Al-Alami Z.M., Bakhtiar A. SARS-CoV-2 journey: from alpha variant to omicron and its sub-variants. Infection. 2024;52(3):767–786. doi: 10.1007/s15010-024-02223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joli J., Buck P., Zipfel S., Stengel A. Post-COVID-19 fatigue: a systematic review. Front Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.947973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N., Dantzer R., Khandaker G.M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology. 2021;131 doi: 10.1016/j.psyneuen.2021.105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Soung A., Sissoko C., et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Review. 2021 doi: 10.21203/rs.3.rs-1031824/v1. [DOI] [Google Scholar]
- Kong K., Chang Y., Qiao H., et al. Paxlovid accelerates cartilage degeneration and senescence through activating endoplasmic reticulum stress and interfering redox homeostasis. J. Transl. Med. 2022;20(1):549. doi: 10.1186/s12967-022-03770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaky blood–brain barrier in long-COVID-associated brain fog. Nat. Neurosci. 2024;27(3):395–396. doi: 10.1038/s41593-024-01577-8. [DOI] [PubMed] [Google Scholar]
- Leng A., Shah M., Ahmad S.A., et al. 2023. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machkovech H.M., Hahn A.M., Garonzik Wang J., et al. Persistent SARS-CoV-2 infection: significance and implications. Lancet Infect. Dis. 2024;24(7):e453–e462. doi: 10.1016/S1473-3099(23)00815-0. [DOI] [PubMed] [Google Scholar]
- Manganotti P., Michelutti M., Furlanis G., Deodato M., Buoite Stella A. Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin. Neurophysiol. 2023;151:83–91. doi: 10.1016/j.clinph.2023.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci M.E., Napoletani G., Protto V., et al. Herpes simplex virus-1 in the brain: the dark side of a sneaky infection. Trends Microbiol. 2020;28(10):808–820. doi: 10.1016/j.tim.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Marogianni C., Sokratous M., Dardiotis E., Hadjigeorgiou G.M., Bogdanos D., Xiromerisiou G. Neurodegeneration and inflammation—an interesting interplay in Parkinson's disease. IJMS. 2020;21(22):8421. doi: 10.3390/ijms21228421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolini C., Kuritzkes D.R., Marra F., et al. Recommendations for the management of drug–drug interactions between the COVID 19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharma and Therapeutics. 2022;112(6):1191–1200. doi: 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurkiewicz I., Chatys Bogacka Z., Slowik J., et al. Fatigue after COVID 19 in non hospitalized patients according to sex. Brain and Behavior. 2023;13(2) doi: 10.1002/brb3.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas S., Jagannathan P., Henrich T.J., et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC) Elife. 2023;12 doi: 10.7554/eLife.86014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss R., Wearden A., Mullis R. Exploring the validity of the chalder fatigue scale in chronic fatigue syndrome. J. Psychosom. Res. 1998;45(5):411–417. doi: 10.1016/S0022-3999(98)00022-1. [DOI] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J. Neurol. Sci. 2021;420 doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID‐19. Euro J of Neurology. 2022;29(6):1652–1662. doi: 10.1111/ene.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M.S., Van Eeden C., Cohen Tervaert J.W. Fatal COVID-19 infections: is NK cell dysfunction a link with autoimmune HLH? Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaan A.A., Al-Ahmed S.H., Garout M.A., et al. Diverse immunological factors influencing pathogenesis in patients with COVID-19: a review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens. 2021;10(5):565. doi: 10.3390/pathogens10050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina J., Iglesias C. Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination. Rev Esp Quimioter. 2022;35(3):236–240. doi: 10.37201/req/002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler C.X., Wyller V.B.B., Moss-Morris R., et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect. Dis. 2021;8(10):ofab440. doi: 10.1093/ofid/ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib K., Goel V., Dubin J.A., VanderDoes J., Butt Z.A. Exploring the syndemic impact of COVID-19 and mental health on health services utilisation among adult Ontario population. Publ. Health. 2024;236:70–77. doi: 10.1016/j.puhe.2024.07.008. [DOI] [PubMed] [Google Scholar]
- Schultheiß C., Willscher E., Paschold L., et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Reports Medicine. 2022;3(6) doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Wang P., Shen J., et al. Neurological Manifestations of hospitalized patients with mild to moderate infection with SARS-CoV-2 Omicron variant in Shanghai, China. Journal of Infection and Public Health. 2023;16(2):155–162. doi: 10.1016/j.jiph.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif Z.A., Gomez C.R., Connors T.J., Henrich T.J., Reeves W.B. RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC) Elife. 2023;12 doi: 10.7554/eLife.86002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaweethai T., Jolley S.E., Karlson E.W., et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C., Cholevas C., Polyzoidis K., Politis A. Long‐COVID syndrome‐associated brain fog and chemofog: luteolin to the rescue. Biofactors. 2021;47(2):232–241. doi: 10.1002/biof.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., et al. In: Madeddu G., editor. vol. 15. 2020. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. (PLoS ONE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Feltz-Cornelis C., Turk F., Sweetman J., et al. Prevalence of mental health conditions and brain fog in people with long COVID: a systematic review and meta-analysis. Gen. Hosp. Psychiatr. 2024;88:10–22. doi: 10.1016/j.genhosppsych.2024.02.009. [DOI] [PubMed] [Google Scholar]
- Varchetta S., Mele D., Oliviero B., et al. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021;18(3):604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhao D., Xiao W., et al. Paxlovid reduces the risk of Long COVID in patients six months after hospital discharge. J. Med. Virol. 2023;95(8) doi: 10.1002/jmv.29014. [DOI] [PubMed] [Google Scholar]
- Witkowski M., Tizian C., Ferreira-Gomes M., et al. Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells. Nature. 2021;600(7888):295–301. doi: 10.1038/s41586-021-04142-6. [DOI] [PubMed] [Google Scholar]
- xin Zhan Y., yu Zhao S., Yuan J., et al. Prevalence and influencing factors on fatigue of first-line nurses combating with COVID-19 in China: a descriptive cross-sectional study. CURR MED SCI. 2020;40(4):625–635. doi: 10.1007/s11596-020-2226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S.J., Liu S. Proposed subtypes of post‐COVID‐19 syndrome (or long‐COVID) and their respective potential therapies. Rev. Med. Virol. 2022;32(4) doi: 10.1002/rmv.2315. [DOI] [PubMed] [Google Scholar]
- Zhang N., Zuo Y., Jiang L., Peng Y., Huang X., Zuo L. Epstein-barr virus and neurological diseases. Front. Mol. Biosci. 2022;8 doi: 10.3389/fmolb.2021.816098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cooper-Knock J., Weimer A.K., et al. Multiomic analysis reveals cell-type-specific molecular determinants of COVID-19 severity. Cell Systems. 2022;13(8):598–614.e6. doi: 10.1016/j.cels.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Shibata K., Hellyer P.J., et al. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Communications. 2022;4(1) doi: 10.1093/braincomms/fcab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.