Abstract
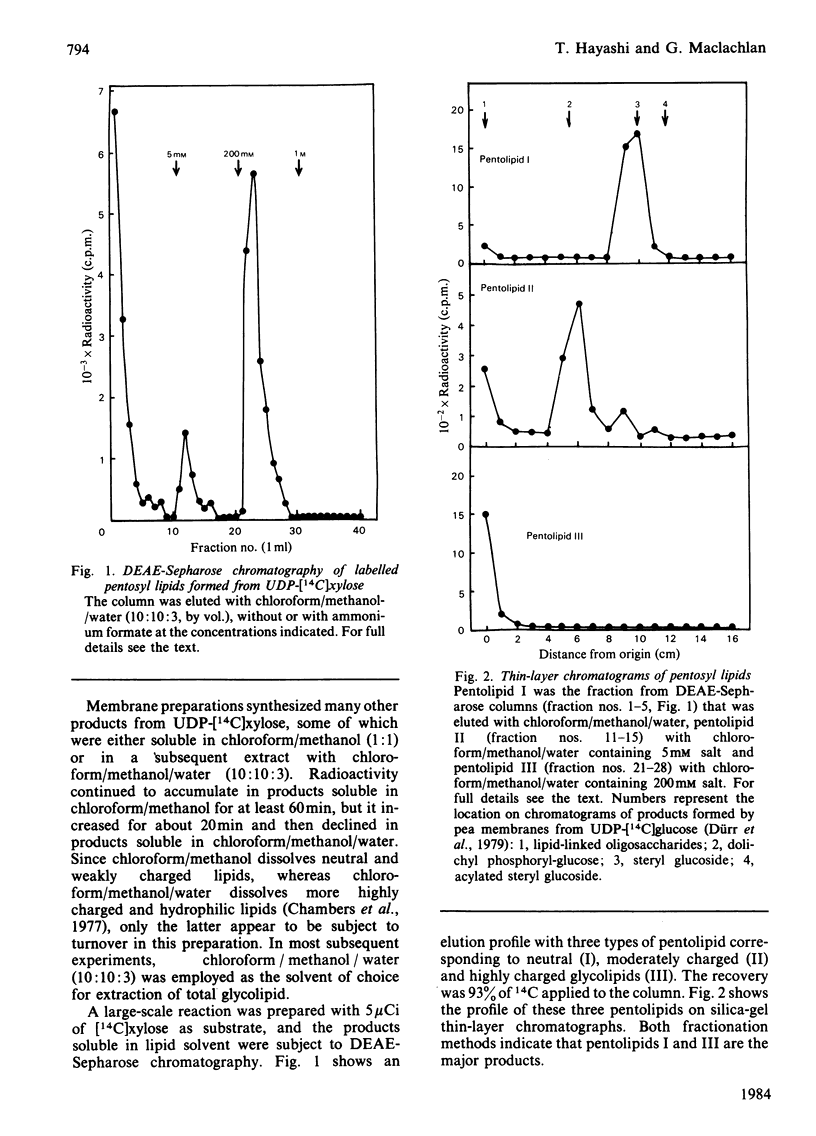
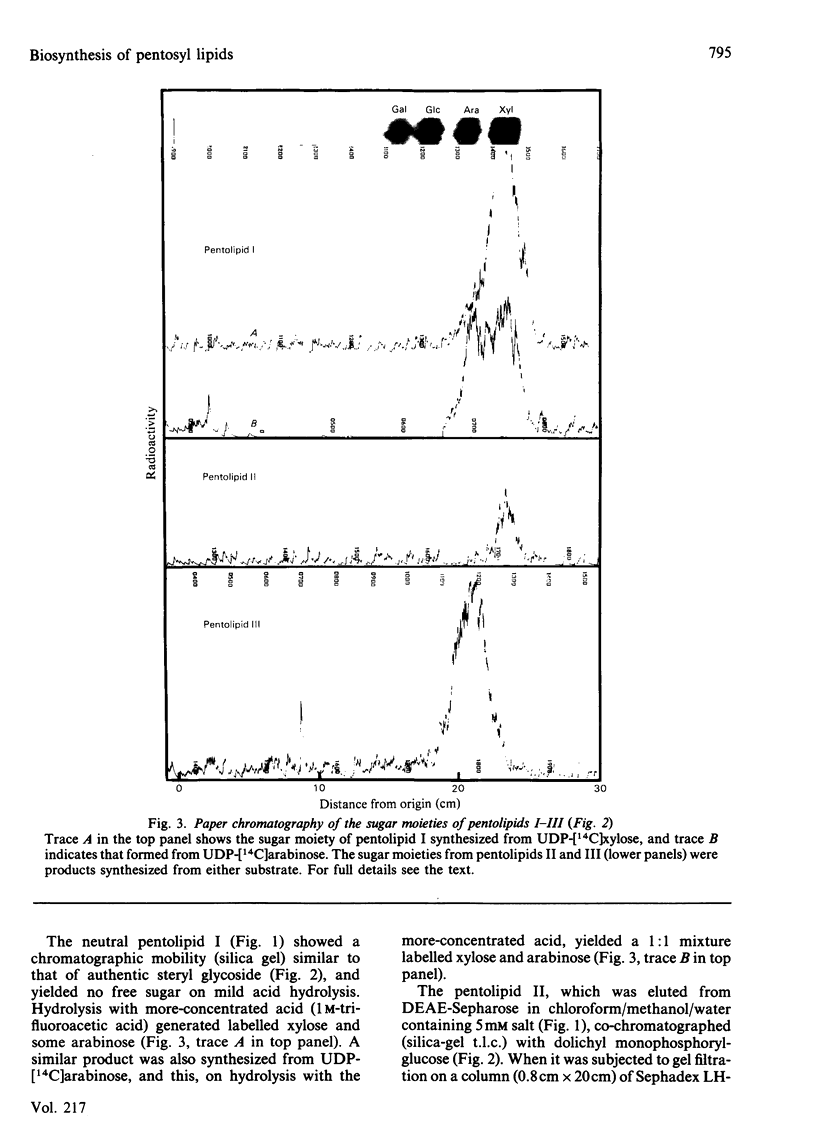
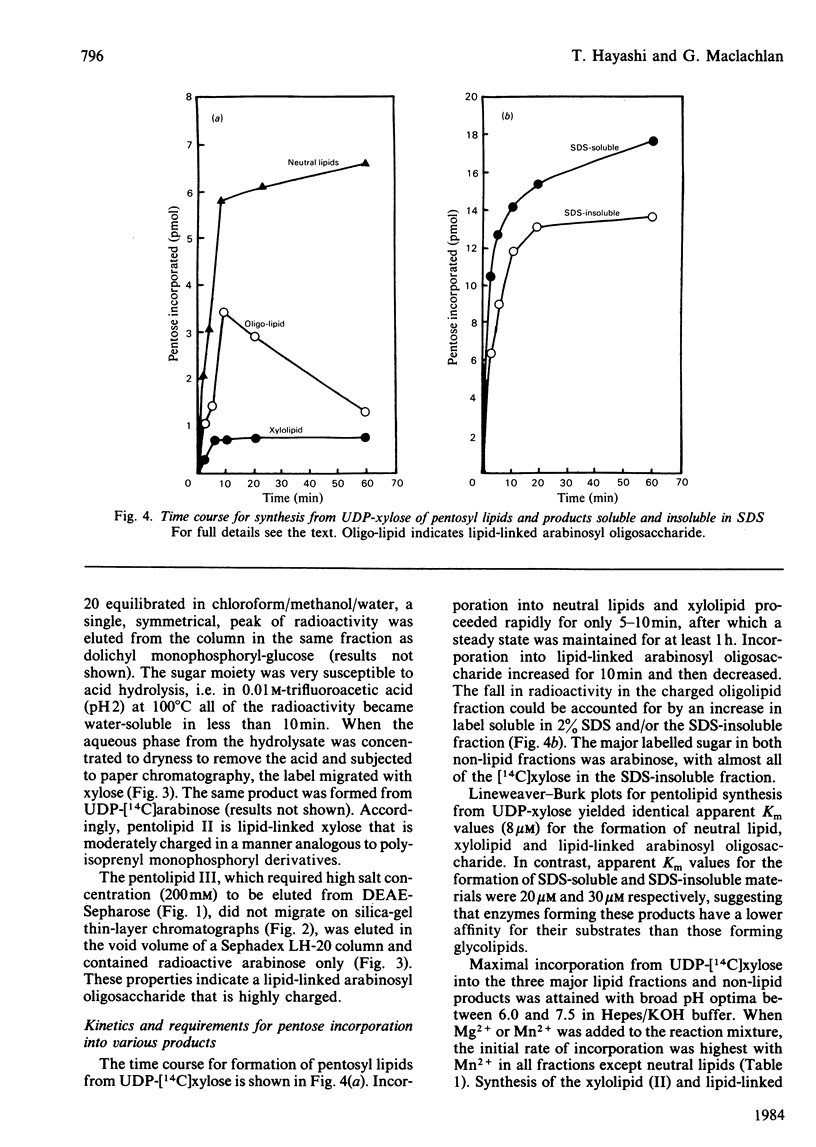
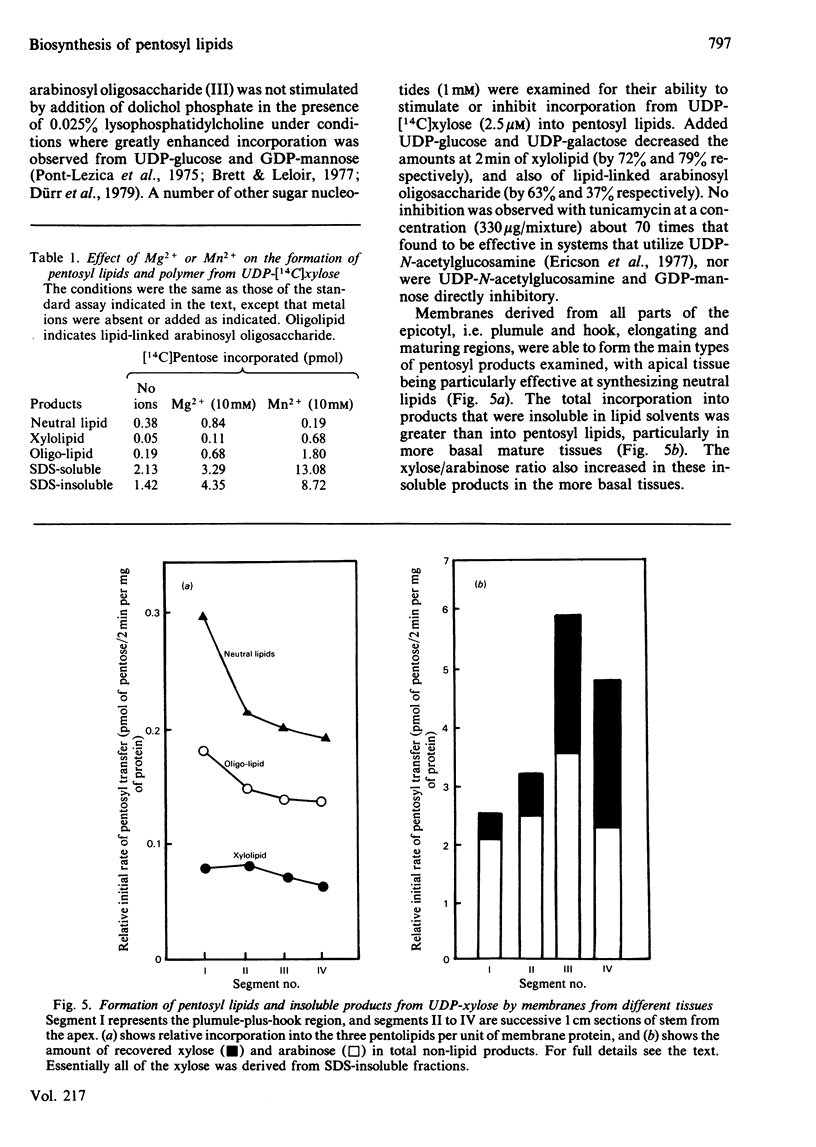
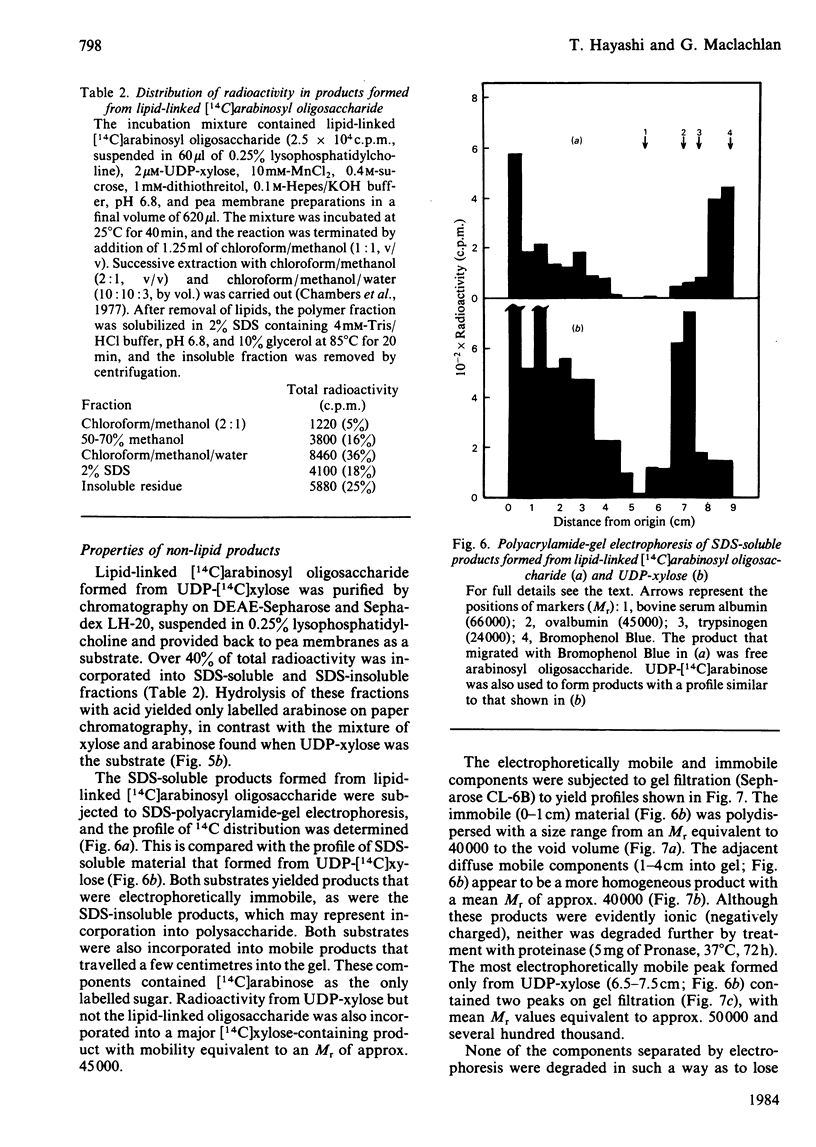
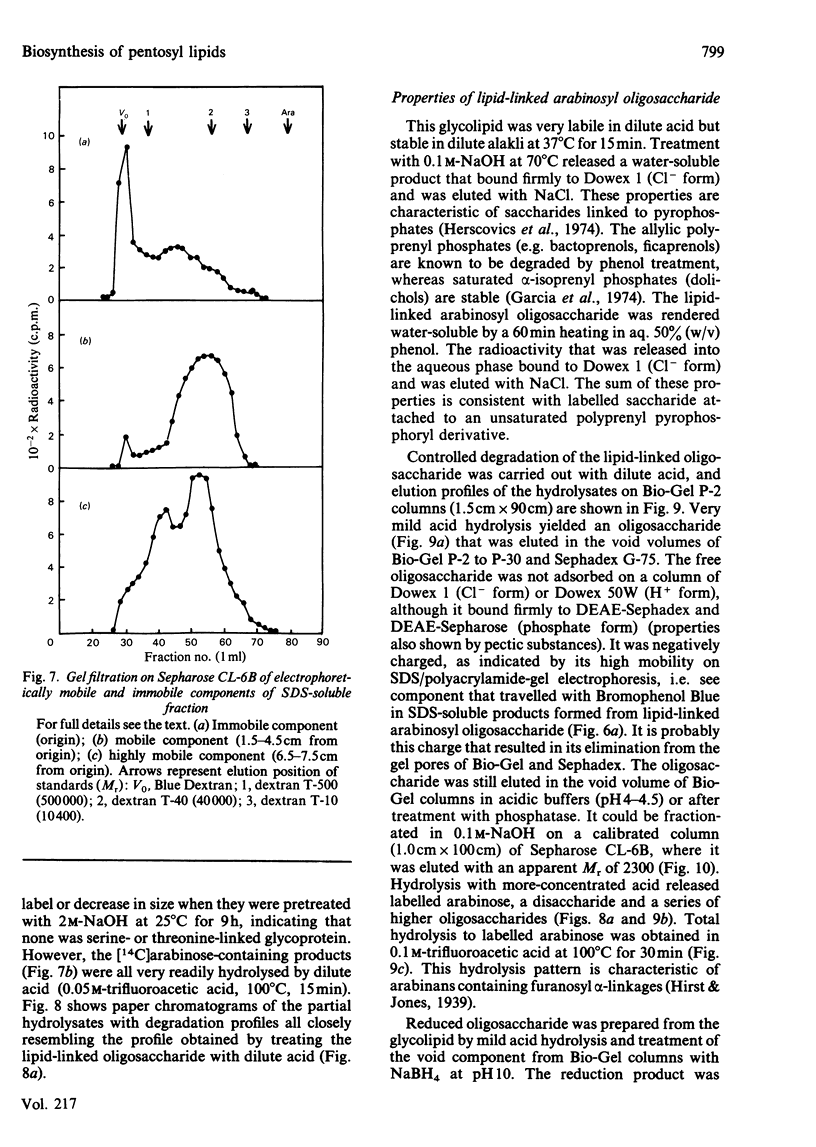
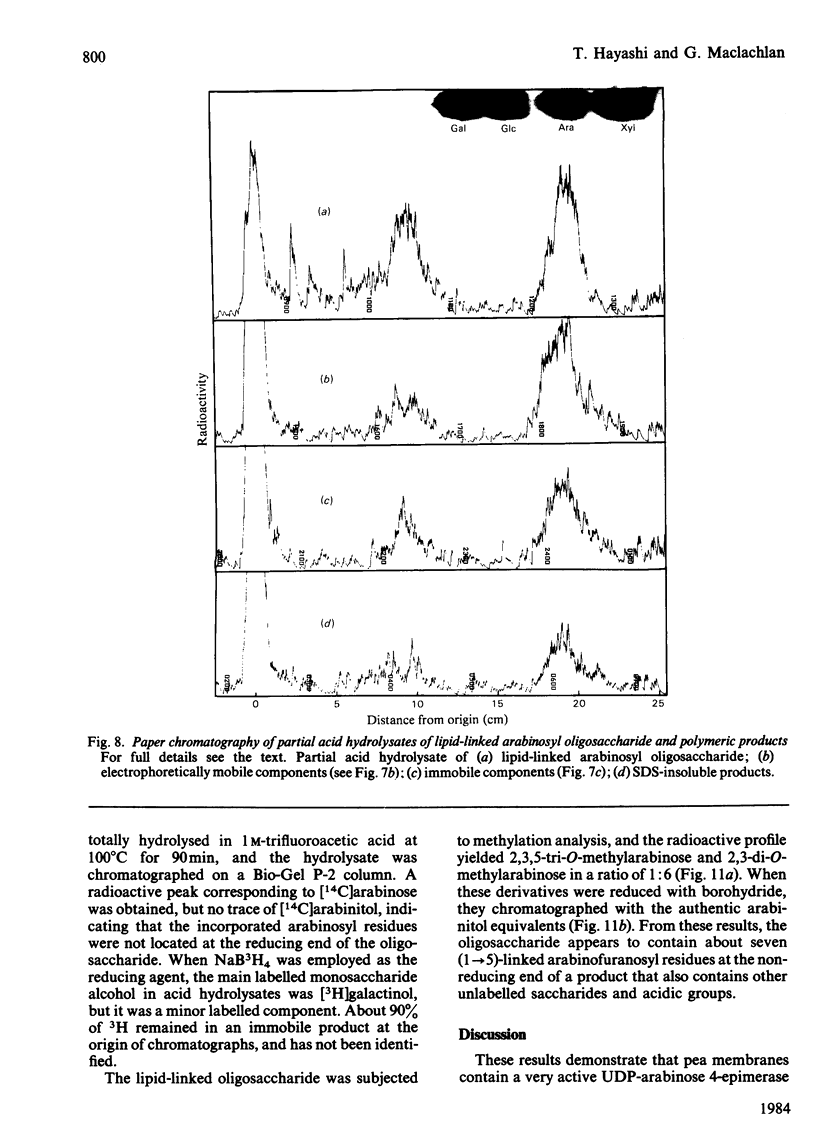
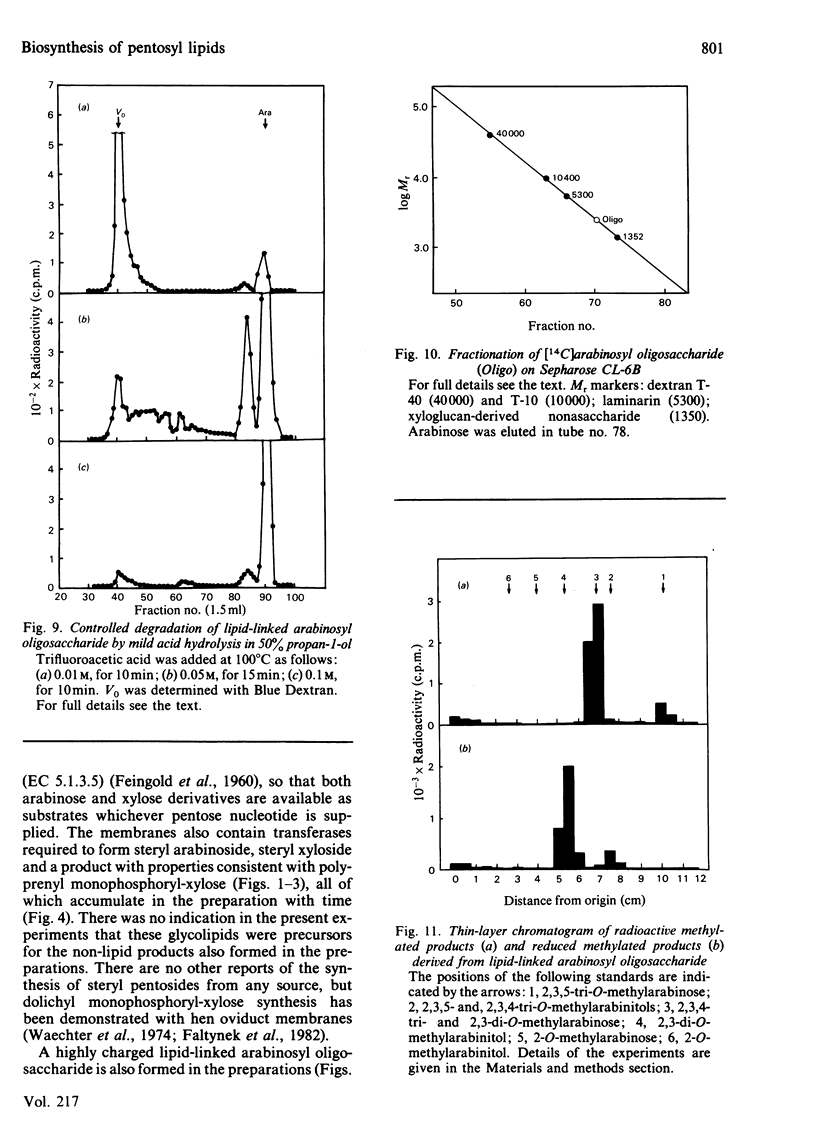
Pea membranes were incubated with UDP-[14C]xylose or UDP-[14C]arabinose and sequentially extracted with chloroform/methanol/water (10:10:3, by vol.) and sodium dodecyl sulphate (2%, w/v). An active epimerase in the membranes rapidly interconverted the two pentosyl nucleotides. Chromatographic analysis of the lipid extract revealed that both substrates gave rise to xylose- and arabinose-containing neutral lipids, xylolipid with properties similar to a polyisoprenol monophosphoryl derivative, and highly charged lipid-linked arabinosyl oligosaccharide. When UDP-[14C]pentose or the extracted lipid-linked [14C]arabinosyl oligosaccharide were used as substrates, their 14C was also incorporating into sodium dodecyl sulphate-soluble and -insoluble fractions as major end products. Polyacrylamide-gel electrophoresis of sodium dodecyl sulphate-soluble products indicated the formation of mobile components with Mr values between 40 000 and 200 000 (Sepharose CL-6B). The lipid-linked [14C]arabinosyl oligosaccharide possessed properties comparable with those of unsaturated polyisoprenyl pyrophosphoryl derivatives. It was hydrolysed by dilute acid to a charged product (apparent Mr 2300) that could be fractionated in alkali. It was degraded to shorter labelled oligosaccharides by slightly more concentrated acid and eventually to [14C]arabinose as the only labelled component. Susceptibility to acid hydrolysis, and methylation analysis, indicated that the oligosaccharide contained approximately seven sequential alpha-1,5-linked arabinofuranosyl units at the non-reducing end. Several acidic residues appear to be interposed between the terminal arabinosyl units and the charged lipid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. S., Deluca V., Dürr M., Verma D. P., Maclachlan G. A. Involvement of Lipid-linked Oligosaccharides in Synthesis of Storage Glycoproteins in Soybean Seeds. Plant Physiol. 1980 Dec;66(6):1113–1118. doi: 10.1104/pp.66.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. S., Dürr M., Burke J., Maclachlan G. The assembly of lipid-linked oligosaccharides in plant and animal membranes. J Supramol Struct. 1979;11(2):123–138. doi: 10.1002/jss.400110203. [DOI] [PubMed] [Google Scholar]
- Bailey R. W., Hassid W. Z. Xylan synthesis from uridine-diphosphate-d-xylose by particulate preparations from immature corncobs. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1586–1593. doi: 10.1073/pnas.56.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Arabinan synthase and xylan synthase activities of Phaseolus vulgaris. Subcellular localization and possible mechanism of action. Biochem J. 1983 Feb 15;210(2):497–507. doi: 10.1042/bj2100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C. T., Leloir L. F. Dolichyl monophosphate and its sugar derivatives in plants. Biochem J. 1977 Jan 1;161(1):93–101. doi: 10.1042/bj1610093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C. T., Northcote D. H. The formation of oligoglucans linked to lipid during synthesis of beta-glucan by characterized membrane fractions isolated from peas. Biochem J. 1975 Apr;148(1):107–117. doi: 10.1042/bj1480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMINATTI H., PASSERON S., DANKERT M., RECONDO E. SEPARATION OF SUGAR NUCLEOTIDES, PHOSPHORIC ESTERS AND FREE SUGARS BY PAPER CHROMATOGRAPHY WITH SOLVENTS CONTAINING BORATES OF ORGANIC BASES. J Chromatogr. 1965 May;18:342–348. doi: 10.1016/s0021-9673(01)80372-1. [DOI] [PubMed] [Google Scholar]
- Chambers J., Forsee W. T., Elbein A. D. Enzymatic transfer of mannose from mannosyl-phosphoryl-polyprenol to lipid-linked oligosaccharides by pig aorta. J Biol Chem. 1977 Apr 25;252(8):2498–2506. [PubMed] [Google Scholar]
- Delmer D. P., Kulow C., Ericson M. C. Glycoprotein Synthesis in Plants: II. Structure of the Mannolipid Intermediate. Plant Physiol. 1978 Jan;61(1):25–29. doi: 10.1104/pp.61.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Bailey D. S., MacLachlan G. Subcellular distribution of membrane-bound glycosyltransferases from pea stems. Eur J Biochem. 1979 Jul;97(2):445–453. doi: 10.1111/j.1432-1033.1979.tb13132.x. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Gafford J. T., Elbein A. D. Tunicamycin inhibits GlcNAc-lipid formation in plants. J Biol Chem. 1977 Nov 10;252(21):7431–7433. [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. The 4-epimerization and decarboxylation of uridine diphosphate D-glucuronic acid by extracts from Phaseolus aureus seedlings. J Biol Chem. 1960 Apr;235:910–913. [PubMed] [Google Scholar]
- Faltynek C. R., Silbert J. E., Hof L. Xylosylphosphoryldolichol synthesized by chick embryo epiphyses. Not an intermediate in proteoglycan biosynthesis. J Biol Chem. 1982 May 25;257(10):5490–5495. [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Glycoprotein biosynthesis in plants. Demonstration of lipid-linked oligosaccharides of mannose and N-acetylglucosamine. J Biol Chem. 1975 Dec 25;250(24):9283–9293. [PubMed] [Google Scholar]
- García R. C., Recondo E., Dankert M. Polysaccharide biosynthesis in Acetobacter xylinum. Enzymatic synthesis of lipid diphosphate and monophospate sugars. Eur J Biochem. 1974 Mar 15;43(1):93–105. doi: 10.1111/j.1432-1033.1974.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Clarke A. E. Antigenic determinants of a plant proteoglycan, the Gladiolus style arabinogalactan-protein. Biochem J. 1980 Nov 1;191(2):437–447. doi: 10.1042/bj1910437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hayashi T., Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. Occurrence and some properties of xyloglucan 4-beta-D-glucosyltransferase and 6-alpha-D-xylosyltransferase. J Biol Chem. 1981 Nov 10;256(21):11117–11122. [PubMed] [Google Scholar]
- Herscovics A., Warren C. D., Jeanloz R. W., Wedgwood J. F., Liu I. Y., Strominger J. L. Occurrence of a beta-D-mannopyranosyl phosphate residue in the polyprenyl mannosyl phosphate formed in calf pancreas microsomes and in human lymphocytes. FEBS Lett. 1974 Sep 1;45(1):312–317. doi: 10.1016/0014-5793(74)80869-0. [DOI] [PubMed] [Google Scholar]
- Hopp H. E., Romero P. A., Daleo G. R., Pont Lezica R. Synthesis of cellulose precursors. The involvement of lipid-linked sugars. Eur J Biochem. 1978 Mar 15;84(2):561–571. doi: 10.1111/j.1432-1033.1978.tb12199.x. [DOI] [PubMed] [Google Scholar]
- Karr A. L. Isolation of an enzyme system which will catalyze the glycosylation of extensin. Plant Physiol. 1972 Aug;50(2):275–282. doi: 10.1104/pp.50.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Fartaczek F., Tanner W., Kauss H. Formation of polyprenol-linked mono- and oligosaccharides in Phaseolus aureus. Arch Biochem Biophys. 1976 Aug;175(2):419–426. doi: 10.1016/0003-9861(76)90529-4. [DOI] [PubMed] [Google Scholar]
- Lezica R. P., Brett C. T., Martinez P. R., Dankert M. A. A glucose acceptor in plants with the properties of an alpha-saturated polyprenyl-monophosphate. Biochem Biophys Res Commun. 1975 Oct 6;66(3):980–987. doi: 10.1016/0006-291x(75)90736-6. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Richardson N. G. Polysaccharides in germination. Occurrence, fine structure, and possible biological role of the pectic araban in white mustard cotyledons. Biochemistry. 1966 Oct;5(10):3099–3107. doi: 10.1021/bi00874a003. [DOI] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lucas J. J., Lennarz W. J. Evidence for xylosyl lipids as intermediates in xylosyl transfers in hen oviduct membranes. Biochem Biophys Res Commun. 1974 Jan 23;56(2):343–350. doi: 10.1016/0006-291x(74)90848-1. [DOI] [PubMed] [Google Scholar]
- Yamagishi T., Matsuda K., Watanabe Y. Characterization of the fragments obtained by enzymic and alkaline degradation of rice-bran proteoglycans. Carbohydr Res. 1976 Aug;50(1):63–74. doi: 10.1016/s0008-6215(00)84083-5. [DOI] [PubMed] [Google Scholar]