Abstract
Terminalia brownii Fresen, an African medicinal plant, is known for its analgesic, antiulcer, and antimicrobial properties, with its leaves, bark, and fruits deeply ingrained in indigenous healing practices. Two lectins, TerBLL (from leaves) and TerBSL (from seeds) of Terminalia brownii Fresen, were purified using salting-out and affinity chromatography on a fetuin-agarose column. The purified lectins were then assessed for protein yield, hemagglutination activity, and physicochemical properties. Both TerBLL and TerBSL have subunits with molecular weights of 57.3 and 65.7 kDa, respectively. TerBLL remains stable at 60–80 °C and is activated by Mn+2, while TerBSL is activated by Zn+2. These lectins maintain consistent activity under acidic conditions, with TerBLL demonstrating heightened activity at extreme alkaline pH. TerBLL retained 50 % of its activity in 2–8M urea, in contrast to the 13 % of TerBSL. Investigation of the properties of TerBLL revealed that it had antinociceptive effects, reducing abdominal pain and prolonging latency time in the hotplate assay, potentially through μ-opioid receptor blockade akin to that of morphine. TerBLL exhibits antiulcer activity at doses of 0.25 and 1 mg/kg, reducing ulcer formation by up to 33 %, comparable to that of pantoprazole (80 mg/kg). The physiochemical attributes of TerBLL, in addition to its pain-relieving and gastroprotective effects, underscore its therapeutic promise, which is consistent with its traditional use.
Keywords: Terminalia brownii, Medicinal plant, Stability, Antinociceptive, Morphine receptors, Gastroprotection, Antiulcer
Graphical abstract
Highlights
-
•
Terminalia brownii is used as medicine for pain relief and ulcer management.
-
•
Two lectins isolated from the leaves and seeds show distinct biological characteristics.
-
•
Terminalia leaf lectin (TerBLL) relieves pain and protects against gastric ulcers.
-
•
TerBLL may inhibit pain by blocking μ-opioid receptors.
-
•
TerBLL holds promise as a potential future analgesic and gastroprotective agent.
1. Introduction
Lectins constitute a distinctive category of (glyco-) proteins that originate outside the immune system and exhibit a specific ability to recognize and form reversible bonds with carbohydrates and glycoconjugates through weak hydrogen bonds, van der Waals forces, and hydrophobic interactions [[1], [2], [3]]. Lectins, which are ubiquitous proteins, agglutinate erythrocytes by binding to carbohydrate antigens on their surfaces. This interaction can be inhibited by the presence of lectin-hapten sugar. Following this ancient discovery, a series of observations revealed that, based on their specificity, lectins can bind to the cellular glycocalyx. This binding, in turn, triggers various cellular activities, such as mitogenesis [4], pain modulation, inflammation, anti-inflammation induction [5], cellular repair stimulation [6], and prevention of stomach lining damage [7]. Traditionally, plant lectins are used in diagnostic applications such as blood typing and cell agglutination tests [8], as well as in studying cell-cell interactions [9]. While their therapeutic applications, including potential uses in cancer therapy, are promising, they still remain under investigation in clinical trials [10]. Terminalia brownii Fresen belongs to the Combretaceae family and is native to tropical and savanna regions of Eastern African countries [11], such as Sudan, where it is locally known as Asha'af [12]. Since ancient times, its medicinal properties have been widely acknowledged in African traditional medicine, and it has been embraced and prescribed by traditional healers to address various health issues. The bark is boiled or chewed to alleviate abdominal pain, while a hot water extract is ingested to relieve persistent joint pain. Furthermore, chewing bark is considered a remedy for both coughs and joint stiffness. As indicated by Mbwambo et al. (2007), the leaves of T. brownii have applications in treating conditions such as heartburn, pain killing, gastric ulcers, stomach ache, and colic [11,[13], [14], [15], [16], [17]].
Antinociceptive drugs are typically classified based on their mechanism of action, distinguishing between those affecting the central nervous system and those targeting the peripheral nervous system [18]. Existing therapies for inflammation and pain management often have side effects and limited efficacy. Consequently, there is a substantial demand for novel and more potent anti-inflammatory compounds [19]. As herbal medicines emerge as promising therapeutic drugs, the exploration of plant lectins has become increasingly important. Beyond their diverse medical applications, many plant lectins have been demonstrated to suppress pain, exhibit anti-inflammatory properties [20,21], and possess gastro-protective effects [7,21]. Consequently, there is a critical need to isolate and purify more lectins from plants. The interest in these proteins is heightened by the fact that many plants known to contain lectins are routinely used by locals for treating various ailments [20] with minimal noticeable side effects. This entails a comprehensive analysis of lectin characteristics and an exploration of the potential link between the previously observed analgesic and antiulcer activities attributed to T. Brownii Fresen by locals and the possible role of lectins in such actions. The motivation for this investigation arises from our laboratory's identification of substantial lectin activity in the aqueous extracts of both the leaves and seeds of the plant. The overarching objective of this study was to isolate and partially characterize the identified lectins and explore their potential in vivo anti-inflammatory, antinociceptive, and antiulcer effects in experimental animals.
2. Methodology
2.1. Chemicals and materials
Most chemicals used are sourced from Sigma-Aldrich (3300 S 2 nd St #3306, St. Louis, MO 63118, United States) or from VWR International GmbH (Graumanngasse 7, 1150 Vienna, Austria). Alternatively, they are obtained from the highest-grade chemical suppliers available.
2.2. Plant materials
High-quality seeds and leaves of Terminalia brownii Fresen were collected from different localities in Khartoum, Sudan. Plant taxonomists at the Herbarium of the National Centre for Research in Khartoum verified the plant, and voucher identification number 2019/05–32 was deposited at the herbarium.
2.3. Determination of protein content
Protein quantification was carried out utilizing the Bradford method at a wavelength of 595 nm and the bicinchoninic acid (BCA) assay at a wavelength of 560 nm, employing bovine serum albumin (BSA) as the standard [22,23].
2.4. Blood samples
Blood samples representing human blood types (A, B, AB, and O) were acquired from healthy donors affiliated with the Department of Biotechnology at the Africa City of Technology and Bahri Teaching Hospital. Animal blood samples, sourced from various animals (cow, sheep, donkey, camel, and goat), were collected from the Faculty of Veterinary Medicine at the University of Khartoum, Sudan.
2.5. Ethical considerations for human and animal subjects
This research protocol was rigorously reviewed and approved by the Africa City of Technology Ethical Review Committee (Approval No: Eth-App-Biotech/12–2019). All blood donor participants were thoroughly informed about the aims, objectives, and potential implications of the project. Prior to their participation, each donor provided informed verbal consent, confirming their understanding and voluntary involvement. Informed consent was also obtained from participants (or their legal guardians) for the publication of any images, clinical data, and other data included in the manuscript.
Blood samples were collected exclusively for hemagglutination tests aimed at evaluating lectin activity and stability, adhering strictly to scientific and ethical standards. Additionally, animal experiments were conducted in accordance with the highest ethical standards for the humane treatment of animals, ensuring their welfare throughout the study. The research complies with all relevant national and international ethical guidelines for both human and animal subjects.
2.6. Extraction of Terminalia brownii lectins
2.6.1. Extraction and fractionation of crude extract (FrA)
A total of 85 g of finely ground powder was produced from meticulously dried, high-quality seeds and leaves using a coffee mill. Subsequently, the obtained powder underwent a 4 h defatting and depigmentation process with petroleum ether (10 mL/g) at room temperature, following the specified method [7]. Following centrifugation and washing with fresh petroleum ether, resulting in a pale greenish color for leaves and a creamy color for seeds, approximately 76 and 80 g of leaf and seed powder, respectively, were obtained. This powder was then subjected to extraction (1 g:10 mL W/V) at 4 °C for 4 h using various buffers, including 20 mM acetate-buffered saline (pH 5), 20 mM PBS (pH 7.4), and 20 mM Tris-HCl (pH 7.5). The extracts were centrifuged at 3220 g for 30 min, and the crude extract supernatant (FrA (FrA-S and FrA-L)) was stored at −20 °C for future use.
2.7. Purification of lectins
Crude extracts (FrA) were subjected to successive incubation with solid ammonium sulfate (AS) at three different concentrations, achieving 60 %, 80 %, and 100 % AS saturation through the salting-out technique following the methodology of Englard and Seifter [24]. The resulting aggregated proteins, captured at specific AS concentrations, were then dissolved in a minimal amount of 20 mM PBS at pH 7.4. Subsequently, the fractionated proteins were subjected to dialysis in 3 kDa tubes against 20 mM PBS, pH 7.4, and the supernatants were stored at −20 °C. To further purify the isolated lectins detected in the fractions and crude extract samples, fetuin-agarose affinity chromatography was employed. In summary, the protein samples were subjected to affinity column chromatography on fetuin-agarose, and protein recycling was carried out at least four times to maximize lectin retention. Unbound protein was washed off with at least 12 bed column volumes of PBS, and the bound lectin was eluted with 3 % acetic acid prepared in 150 mM NaCl. Five-millilitre fractions were collected, and the pH was adjusted to neutral using NaOH (8 M). Samples with lectin activity were pooled, dialyzed, and lyophilized to dryness until further use.
2.8. Hemagglutination activity (HA)
Hemagglutination activity (HA) was determined according to the methods of Awadallah et al. [25]. In a 96-well ELISA U-shaped plate, 50 μL each of crude extracts, fractionated proteins, and purified lectin samples from Terminalia brownii leaves and seeds were evaluated. These samples were twofold serially diluted with 50 μL of their respective buffers. Subsequently, 50 μL of 2 % (V/V) trypsin-treated or untreated human or animal erythrocytes were added, and the mixture was incubated for 1 h at room temperature. The agglutination titre was determined by visual observation. The hemagglutination unit was defined as the reciprocal of the highest dilution of a sample that causes visible agglutination of red blood cells in a standardized assay, while the specific activity was defined as units per milligram of protein (U/mg).
2.8.1. Carbohydrate inhibition assay
Lectin carbohydrate specificity was determined through a hemagglutination inhibition test. To test the inhibition effect, concentrations of 200 mM (D-glucose, D-ribose, N-acetyl galactosamine (GalNAc), D-galactose, D-maltose, and D-mannose) were used, following the protocol outlined by Konozy et al. [26]. In brief, 40 μL of each sugar, with an equal quantity of PBS, was serially diluted. Subsequently, 40 μL of the lectin sample was added. The plate was left at room temperature for 2 h before 40 μL of 2 % erythrocytes was added. The results are expressed as the lowest concentration of carbohydrate (MIC) capable of inhibiting 2 units of lectin activity.
2.9. Characterization of Terminalia brownii leaf lectin (TerBLL) and seed lectin (TerBSL)
2.9.1. SDS‒PAGE
To determine the molecular weights of the subunits of the purified TerBLL and TerBSL proteins, sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) analysis was conducted according to the procedure described by Laemmli [27]. A prestained protein marker, featuring standards at 140, 100, 72, 60, 45, 35, 25, and 20 kDa, served as a reference during the analysis.
2.9.2. Glycoprotein nature assay
The phenol‒sulfuric acid method was employed to confirm the glycoprotein nature of the pure lectin [28].
2.9.3. Effects of metal ions on lectin activity
The impact of divalent metal cations such as Ca+2, Mg+2, Ni+2, Cu+2, Zn+2, and Mn+2 on the purified lectin was investigated. Initially, the protein was treated overnight with 100 mM EDTA, prepared in 20 mM Tri-HCl, pH 7.4, and subsequently thoroughly dialyzed against deionized distilled water to eliminate excess EDTA. A hemagglutination assay was performed using lectin appropriately diluted in 20 mM Tris-HCl pH 7.4. Both untreated lectin (positive control) and EDTA-treated lectin were tested in the presence of specified metal ions. A 2 % erythrocyte suspension was introduced, and lectin activity was assessed as previously described.
2.9.4. Effect of pH on lectin activity
The influence of pH on the hemagglutinating activity of lectins was investigated by incubating suitably diluted purified lectin samples in various 100 mM buffers with different pH values (pH 2.5 to pH 10.5). Hemagglutination was evaluated essentially as described previously [29].
2.9.5. Effects of temperature
The effect of temperature on lectin hemagglutinating activity was studied as shown [25]. Briefly, aliquots of suitably diluted lectin samples were incubated at different temperatures (20–100 °C) for 30 min. The lectin samples were immediately cooled on ice, and hemagglutination was determined.
2.9.6. Stability of lectin activity over a two-month period at room temperature
To assess the stability of TerBLL at room temperature, a sterilized lectin sample (500 μL-64 units) was incubated for 60 days at 25–30 °C ± 2 °C. Aliquots (30 μL) were removed at various time intervals, and hemagglutination assays were subsequently conducted [30].
2.9.7. Stability of lectin at the optimal temperature
To study the stability of lectin at its optimal temperature, 500 μL samples were incubated at their respective optimal temperatures for 2 h. Aliquots of 30 μL were then removed, immediately cooled, and analyzed for hemagglutination (HA) content.
2.9.8. Effect of denaturing agents
The effect of urea on lectin stability was investigated following a previously described procedure [26]. Various urea concentrations, ranging from 1 M to 8 M, were incubated with 30 μL of suitably diluted purified lectin samples for 3 h. The residual activity was subsequently determined, with the untreated lectin sample serving as the control for 100 % activity.
2.9.9. Spectrophotometric determination of tryptophan
This procedure followed the method outlined by Spande and Witkop [31]. The tryptophan residues of TerBSL were selectively modified by using the oxidizing reagent N-bromosuccinimide (NBS). TerBSL (0.42 mg/mL) was prepared in 20 mM acetate buffer (pH 6.0) and titrated with freshly prepared NBS (10 mM). Aliquots of the TerBSL solution were added in increments of 20 μL each, with shaking and incubation for 10 min. The solution was then monitored for a decrease in absorbance at 280 nm. The percentage of modified tryptophan residues was determined using the following formula:
where:
ΔOD: corrected optical density decreases at 280 nm; 1.31: absorption factor to convert optical density at 280 nm; V: initial volume of lectin (mL); W: weight of titrated protein (mg); 5500: molar extinction coefficient of tryptophan at 280 nm; 186: molecular weight of bound tryptophan residue.
2.10. Biological activity of TerBLL
2.10.1. Experimental animals
Male Swiss white mice (10 weeks of age) weighing 20 ± 5 g and male Swiss white rats (age 10 weeks at the beginning of the experiment and weighting 120–130 ± 5 g were obtained from the faculty of veterinary medicine at the University of Khartoum, Sudan. Animals were maintained under controlled temperatures on 12-h light/12-h dark cycles with free access to food and water. Mice were allowed to acclimate for 5 days before the experiment. All experimental protocols were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals [32]. The study was approved by the Ethics Review Committee of the Africa City of Technology (ACT), Sudan, ref: Eth-App-Biotech/12–2019 (Supplementary file S1). The animals were individually placed into distinct cages, and food access was restricted, while they had free access to water for a minimum of 12 h before the experiment.
2.10.2. Abdominal writhing assay
The antinociceptive activity of TerBLL was determined according to Koster et al. [33]. The experimental animals were divided into 5 groups (5 mice/group; 20 ± 5 g): G1 received 0.7 % acetic acid (10 μL/g, i.p.) and was considered a negative control; G2–G4 received (3, 6, 12 mg/kg, i.p.) of TerBLL lectin; and G5 received diclofenac Na (10 mg/kg, i.p.) as a reference drug (positive control). After 30 min, the animals in groups G2–G5 received 0.7 % acetic acid. The number of writhes or contractions were determined by abdominal muscle contractions, and hind paw extension was recorded after 20 min, considering the first 10 min to indicate normal reflux of the immune system toward chemical stimuli. The reduction in the number of writhes in animals pretreated with lectin was statistically compared to that in the negative control animals, and the percentage of pain inhibition was calculated.
2.10.3. Hotplate assay
The test was performed according to previous methods [34,35]. The animals were divided into 5 groups, each consisting of 5 mice (20 ± 5 g) (. G1 (negative control group) received saline (0.2 mL/mouse, i.p.). TerBLL was administered to the G1–G4 groups at different doses (3, 6, and 12 mg/kg, i.p.). The final positive control group (G5) received diclofenac Na (10 mg/kg, i.p.). The mice were individually placed on a hotplate (50 ± 5 °C) twice. During the initial trial, any animal displaying a reaction time greater than 20 s was excluded. In the second trial, the reaction or latency time (licking of hind paw or jumping) was recorded at time zero, followed by measurements at 30, 60, and 90 min after administration, with a cut-off time of 40 s. The increase in latency time in lectin-pretreated animals was compared to that in negative control animals. To investigate the potential involvement of opioid receptors, the following groups were prepared: G2 (TerBLL, 12 mg/kg), G3 (TerBLL (12 mg/kg), treated with naloxone (1.5 mg/kg) after 15 min of lectin administration), G4 (morphine (10 mg/kg, i.p.), treated with naloxone (1.5 mg/kg) after 15 min of morphine administration), and G5 (10 mg/kg, i.p.).
2.10.4. Gastroprotective activity of TerBLL
Five male Swiss rats (120–130 ± 5 g) from each of the six groups were deprived of food overnight but had free access to water prior to the experiment. G1 (negative control) received 0.2 mL of 99.9 % ethanol, G2 (positive control) received pantoprazole (80 mg/kg), G3–G5 received different concentrations of TerBLL (0.25, 0.5, or 1 mg/kg, i.p.), and 3 mg/mL normal saline was given to G6. After 30 min, 0.2 mL of ethanol was given to all members of G2–G5, and the animals were left for another 30 min. Then, all groups were euthanized [7]. Ulcer scoring was conducted after incising the stomach along the greater curvature. The number of ulcers per stomach was recorded, and the dimensions of hemorrhagic or ulcerative lesions were assessed and juxtaposed with the overall stomach area. The ulcer index (Ui) was calculated as follows [36]:
where Un is the average number of ulcers per animal, Us is the average number of severity scores of the ulcers (0: normal-colored stomach, 1: spot ulcer, 1.5: hemorrhagic streak, 2: deep ulcer, and 3: perforations), and Up is the percentage of animals with ulcers. The percentage of ulcer inhibition (UI%) was calculated using the following equation:
The pH of the stomach juice was determined utilizing a pH meter.
2.11. Statistical analysis
Descriptive analysis and analysis of variance (one-way ANOVA) were used to analyse the data obtained for the biological effect of TerBLL. Tukey's HSD was used for post hoc tests for mean difference comparisons at a P value of 0.05. The statistical analysis was performed using SPSS software version 26 (IBM SPSS Statistics for Windows, v26.0, IBM Corp, Armonk, NY, USA).
Ethical Clearance.
3. Results
3.1. Extraction and purification
Three equal batches of defatted leaf powder (TerBL) were subjected to extraction with three different buffers (phosphate-buffered saline (PBS), acetate, and Tris-HCl). The protein content and hemagglutination (HA) of each sample were estimated. The highest protein content was achieved with acetate buffer, surpassing the other two buffers, albeit with very low lectin activity. Conversely, the lectin-specific activity was at its maximum with PBS, despite the low protein concentration, indicating the unsuitability of the acetate buffer for lectin extraction. Hence, PBS was deemed suitable for subsequent purification of T. brownii lectins (Fig. 1A). Initially, the crude protein extract underwent salting-out for protein fractionation using ammonium sulfate (AS) salt. The leaf (FrA-L) and seed (FrA-S) crude extracts fractionated with ammonium sulfate exhibited interesting results. The protein content in the dialysate fractions decreased concurrently with a notable increase in lectin-specific activity, which was attributed to the removal of proteins with a molecular weight below the dialysis tube cut-off value (3 kDa). The lectin activity within the crude extract was unevenly distributed among the various protein fractions obtained at different concentrations of ammonium sulfate, with the highest specific activity observed after dialysis in TerBL-Fr80 % (4399.2 U/mg) and TerBS-Fr100 % (406.8 U/mg) (Fig. 1B and C). Incubation of FrA-S and FrA-L extracts with various carbohydrates at concentrations up to 200 mM did not result in any apparent inhibition of lectin hemagglutination (HA) activity (Supplementary File S1: Table S1). This indicates a lack of simple sugar specificity for the lectin and instead suggests specificity towards complex carbohydrate moieties. Consequently, fetuin, a heptagon-synthesized glycoprotein found in the bloodstream containing sialylated N-linked and O-linked glycans, was effectively employed in this study and for various other lectins with complex sugar specificity, facilitating the one-step purification of carbohydrate-binding proteins [37,38]. The loading of the crude extract and AS fractions resulted in the complete retention of lectin during the first load, with no activity detected in the effluent. This finding suggested the suitability of fetuin-agarose as an affinity matrix for lectin purification. This strong interaction was reversed by a low pH of 3 % acetic acid. Low pH solutions are known to deactivate some lectins by distorting their secondary and tertiary structures [39]. Therefore, the complete loss of hemagglutination activity of the lectin in the presence of acetic acid is likely due to the depletion of metal ions resulting from the deactivation by low pH acetic acid. Indeed, when lectin activity was tested following thorough dialysis against deionized distilled water, no lectin activity was observed. However, the addition of divalent metal ions (Mn+2 and Ca+2) successfully restored the activity. Although the purification fold of TerBLL was greater than that of TerBSL, the yield of the latter lectin was notably greater than the former (Table 1).
Fig. 1.
Protein extraction of Terminalia brownii from seeds and leaves. A) The concentration and specific activity of Terminalia brownii leaf and seed lectins (TerBLL and TerBSL, respectively) using different extraction buffers. B) The concentration and specific activity of ammonium sulfate fractionated crude extract from leaves before and after fractions' dialysis. C) The concentration and specific activity of ammonium sulfate fractionated crude extract from seeds before and after fractions' dialysis.
Table 1.
Purification of Terminalia brownii leaf and seed lectins (TerBLL and TerBSL, respectively). The O+ blood group was used for all TerBLL assays, while AB+ was used for TerBSL.
| Lectin source | Purification steps | Total volume (ml) | ●Total protein concentration (mg/ml) | Activity (HU) | aSpecific activity (HU/mg/ml) | Yield (%) | Purification fold |
|---|---|---|---|---|---|---|---|
| TerBLL | Crude (FrA-L) | 30 | 5.16 | 15360 | 2976.7 | 100 | 1 |
| Fetuin-agarose affinity | 3 | 0.474 | 768 | 16203 | 5 | 5.5 | |
| TerBSL | Crude (FrA-S) | 14 | 6.2 | 3584 | 575.3 | 100 | 1 |
| Fetuin-agarose affinity | 2 | 0.196 | 512 | 2612.2 | 14.3 | 4.5 |
●The protein concentration of the leaf extract was estimated using the Bradford assay, while that of the seed extract was estimated by the BCA assay.
Specific activity is defined as the hemagglutination unit (HU) divided by the protein concentration (mg/mL) of the assay solution.
3.2. Hemagglutination activity
Testing the specificity of ammonium sulfate-precipitated protein fractions and affinity-purified lectins for human and animal blood yielded the following results: TerBL-Fr100 % displayed remarkably high activity with a trypsin-treated A blood group (512 U), while TerBL-Fr80 % exhibited a similar notable preference with a trypsin-treated B blood group (512 Unit). Fascinatingly, almost all TerBS-Fr100 % exhibited no lectin activity against human blood, and only a minimal activity of 2 units was detected with TerBS-Fr40 % against the AB blood group. TerBS-Fr80 % demonstrated activity in all blood groups, whether trypsin-treated or untreated, except for the untreated A and B blood groups. Due to the limited availability of animal blood and low concentrations of seed lectin obtained from extraction and fractionation, only the TerBL fractions were tested. Fr80 % and Fr100 % agglutinated only cow and camel blood, with slight increases in activity reported against trypsin-treated erythrocytes (Table 2).
Table 2.
Hemagglutination activity of Terminalia brownii leaf and seed lectins (TerBLL and TerBSL, respectively) ammonium sulfate fractions against trypsin-treated and untreated human and animal erythrocytes.
| Blood groups | TerBLL activity (U) |
TerBSL activity (U) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr60 % |
Fr80 % |
Fr100 % |
Fr60 % |
Fr80 % |
Fr100 % |
|||||||
| ●UT | ∗T | UT | T | UT | T | UT | T | UT | T | UT | T | |
| A | 4 | 16 | 2 | 2 | 4 | 512 | ♦NA | NA | NA | 8 | NA | NA |
| B | 4 | 4 | 8 | 512 | 4 | 4 | NA | NA | NA | 16 | NA | NA |
| AB | 4 | 8 | 4 | 8 | 4 | 4 | 2 | NA | 16 | 16 | NA | NA |
| O | 4 | 4 | 4 | 4 | 4 | 4 | NA | NA | 16 | 8 | NA | NA |
| Cow | NA | NA | NA | 4 | 4 | 16 | – | – | – | – | – | – |
| Goat | NA | NA | NA | NA | NA | NA | – | – | – | – | – | – |
| Sheep | NA | NA | NA | NA | 4 | 8 | – | – | – | – | – | – |
| Donkey | NA | NA | NA | 2 | NA | 4 | – | – | – | – | – | – |
| Camel | NA | NA | 2 | 4 | 4 | 8 | – | – | – | – | – | – |
●UT: Untrypsinated, ∗T: Trypsin treated, ♦NA: No Activity, ⸺: Not performed.
3.3. Physicochemical characterization
Treating TerBLL and TerBSL with EDTA resulted in the complete elimination of their hemagglutination. The addition of divalent metals effectively restored their loss of activity. Interestingly, the two lectins displayed distinct behaviors in response to the addition of divalent metals. Zn+2 and Cu+2 fully restored 100 % of the TerBSL activity, while only limited activity was restored with Ca+2, Mn+2, and Mg+2. For EDTA-inactivated TerBLL, Mn+2 reactivated the lectin by more than 150 % of its original activity (Fig. 2A). TerBLL demonstrated activity within the pH range of 2.5–3.5, after which it began to decrease. However, a sudden sharp increase was observed at pH values between 9.5 and 10.5. The lectin exhibits remarkable activity and operates optimally at pH 9.5, suggesting its potentially unique biological significance. It is noteworthy that at such a highly alkaline pH, the stability and activity of most proteins typically decrease. However, TerBLL maintained consistent activity (Fig. 2B). Upon subjecting aliquots of TerBLL and TerBSL to various temperature intervals ranging from 20 to 100 °C, we observed distinct responses to temperature variations. TerBSL exhibited remarkable stability even at temperatures reaching 100 °C, with two temperature optima observed at 30 °C and a broader range spanning from 70 to 100 °C. Conversely, TerBLL displayed a singular optimum at approximately 40 °C, with activity declining beyond this point, experiencing an approximately 60 % reduction at approximately 100 °C (Fig. 2C). A sterilized TerBLL sample incubated at room temperature (25 ± 3 °C) for 60 days demonstrated constant lectin activity until day 60, after which the lectin activity decreased by 50 % (Supplementary File S1: Fig. S1), indicating the high stability of TerBLL. When both lectins (TerBLL and TerBSL) were incubated for 120 min at their respective optimum temperatures, they remained remarkably stable with minimal reduction in activity from their original levels (Supplementary File S1: Fig. S2). The remarkable stability of these lectins is likely due to their glycoprotein nature. It has been established that glycan chains enhance protein thermal stability, and removing these glycans through deglycosylation significantly decreases protein stability [40,41].
Fig. 2.
Physiochemical characterization of Terminalia brownie leaves and seed lectins (TerBLL and TerBSL, respectively). A) Effect of chelating agent (EDTA) on lectins activity and the subsequent addition of metal ions on the activity. B) Effect of different pH buffers on lectins activity. C) Effect of temperature on lectins activity. D) Residual activity of lectins after incubation with different concentrations of denaturing agent (Urea). E) Oxidization of exposed tryptophan residues from seed lectin TerBSL using N-bromosuccinimide (NBS). F) SDS-PAGE for TerBLL and TerBSL.
To assess the stability of TerBLL and TerBSL in the presence of strong denaturing agents such as urea, incubation experiments were conducted with varying urea concentrations. Both lectins exhibited different levels of stability during the experiment. TerBLL maintained a constant activity of 100 % when subjected to urea concentrations ranging from 1 M to 2 M but exhibited a decrease of more than 50 % in activity at concentrations of 2 M and above. In contrast, TerBSL lost nearly 90 % of its activity when exposed to 1 M urea, with low activity maintained up to 8 M urea (Fig. 2D). When subjected to SDS-PAGE, both lectins TerBLL and TerBSL were observed to separate into two subunits with molecular weights of 57.3 kDa and 65.7 kDa, respectively (Fig. 2F).
Subjecting TerBSL to the tryptophan-specific oxidizing agent N-bromosuccinimide (NBS) resulted in a significant reduction in the OD at 280 nm, indicating tryptophan oxidation. The greatest quenching of TerBSL-tryptophan absorbance at 280 nm occurred upon the addition of 0.8 mg/mL NBS, followed by a sudden increase in absorbance (OD) at a concentration of almost 1 mg/mL NBS (Fig. 2E). This was attributed to the bromination reaction [31]. The calculation of the number of modified tryptophan residues suggested that approximately 2 % of the total exposed tryptophan residues were modified.
3.4. Biological activity of TerBLL
3.4.1. Antinociceptive properties of TerBLL
The antinociceptive properties of the TerBLL were assessed through two classical nociception models. Initially, the chemical model utilized acetic acid to evaluate peripheral antinociception [42], while the thermal hotplate technique was employed to judge pain perception, involving both peripheral and central nervous system pathways. In the acetic acid test, pain is induced by injecting an irritant, such as acetic acid, into the animal peritoneal cavity, leading to characteristic stretching (writhing) movements. The quantifiable number of episodes allows for the assessment of analgesic effects, with analgesics aiming to reduce these episodes. In this study, mice were intraperitoneally administered various doses of TerBLL (3, 6, and 12 mg/kg), followed by exposure to the chemical irritant acetic acid. Although all the doses exhibited significantly different levels of pain relief, the moderate dose (6 mg/kg) led to a noteworthy decrease in abdominal writhing (0.0 ± 0.0, P0.05 = 0.001, 95 % CI = 17.9–82.4), achieving up to 100 % pain relief, comparable to the effect produced by the positive control (diclofenac-Na) (Table 3).
Table 3.
Terminalia brownii leaf lectin (TerBLL) antinociceptive effect and pain relief caused by intraperitoneal administration of acetic acid (0.7 %).
| Groups |
Dose |
Average mice weight |
Abdominal writhing |
Pain inhibition |
One-Way ANOVA |
Tuckey HSDa |
||
|---|---|---|---|---|---|---|---|---|
| (n = 5) | (mg/Kg) | (kg) | (M ± S.D.) | (%) | F | P value | P value | 95 % CI |
| G1 | Acetic acid (0.7 %) | 25.26 ± 4.2 | 50.2 ± 32.4 | 0.0 | 7.428 | 0.001 | - | - |
| G2 | TerBLL (3 mg/kg) | 27.82 ± 4.1 | 12.3 ± 16.9 | 75.4 | 0.016 | 5.7–70.1 | ||
| G3 | TerBLL (6 mg/kg) | 30.02 ± 2.3 | 0.0 ± 0.0 | 100 | 0.001 | 17.9–82.4 | ||
| G4 | TerBLL (12 mg/kg) | 28.12 ± 3.1 | 9.8 ± 10.8 | 80.5 | 0.010 | 8.2–72.6 | ||
| G5 | Diclofenac Na (10) | 24.72 ± 4.2 | 0.0 ± 0.0 | 100 | 0.001 | 17.9–82.4 | ||
Post hoc analysis was performed at the 0.05 significance level; comparisons were performed between G1 (the negative control) and the test groups (G2 – G5); bold numbers represent significant results at P0.05.
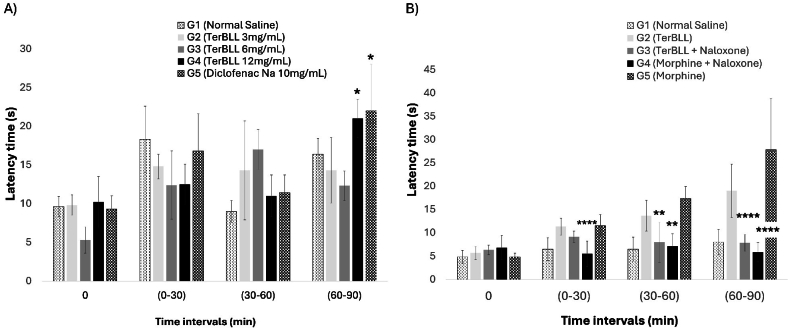
The experiment assessing thermal-induced pain alleviation by TerBLL involved administering TerBLL at various doses (3, 6, and 12 mg/kg) and measuring the latency time on the hotplate to assess its analgesic effect. The results showed that mice in Group 4 treated with 12 mg/kg TerBLL for 90 min experienced a significant reduction in pain perception (Fig. 3-A). The mechanism by which TerBLL alleviates heat-induced pain in the hotplate test was investigated using morphine in conjunction with its antagonist naloxone. Administration of naloxone before TerBLL treatment completely negated the analgesic effect of lectin in this study. Naloxone demonstrated the potential to significantly block the effect of TerBLL, with its impact becoming evident at 60 min and persisting until 90 min postadministration (Fig. 3B). Furthermore, administering naloxone before morphine injection markedly diminished the analgesic effect of morphine.
Fig. 3.
Terminalia brownii leaves lectin (TerBLL) hotplate assay. A) Latency response (Mean (sec) ± S.D.) at different time intervals of rats after administration of leaves lectin TerBLL to pain induced by high temperature; ∗ TerBLL (12 mg/mL) significantly increased the latency time for rats to start licking their paws compared to the negative control (G1); (P0.05 = 0.0170). B) Latency response of pain (Mean (sec) ± S.D.) at different time intervals. ∗: G3 and G4 groups which were treated with naloxone to reverse analgesia showed a significant decrease in pain latency at time intervals (30–90 min) at P0.05.
3.4.2. The gastroprotective effect of TerBLL
To investigate the potential gastroprotective effect of T. brownii leaf lectin (TerBLL), experimental animals were treated with 0.25, 0.5, or 1 mg/kg lectin, followed by the induction of ulcers with ethanol. The findings demonstrated significant effectiveness in reducing the ulcer index for both the 0.25 mg/kg and 1 mg/kg doses of TerBLL (Ui = 6.8 ± 0.12, P < 0.05 = 0.000, 95 % CI = 3.17–3.62), with both doses offering approximately 33.4 % protection against ethanol-induced stomach ulceration. These outcomes are promising and comparable to those of the positive control pantoprazole, which provided 33.6 % protection against ethanol-induced ulceration. Upon testing the gastric juice of animals treated with lectin, notable increases in gastric juice pH were observed, particularly at the 0.25 mg/kg dose, which significantly conferred 33.4 % protection against ethanol-induced ulceration. Comparable patterns were observed with the 0.5 mg/kg dose, although it did not yield significant protection against ethanol-induced ulcers. Surprisingly, the dose of 1 mg/kg G5, which also offered significant protection, induced only a slight increase in gastric pH compared to that of the negative control (saline alone) (Table 4).
Table 4.
The gastric juice pH and the antiulcer effect of Terminalia brownii leaf lectin (TerBLL) on ethanol-induced gastric lesions.
| Groups |
Dose |
pH |
Lesions Un |
Ulcer index |
Ulcer inhibition |
One-Way ANOVAa |
Tuckey HSD∗∗ |
||
|---|---|---|---|---|---|---|---|---|---|
| (n = 5) | (mg/kg) | M ± S.D. | (mm2) | Ui | UI% | F | P value | P value | 95 % CI |
| G1 | Ethanol (99.9 %) | 6.0 ± 0.0 | 0.32 ± 0.1 | 10.2 ± 0.03 | 0.0 | 6156.3 | 0.000 | – | – |
| G2 | Pantoprazole | 6.3 ± 0.6 | 0.16 ± 0.1 | 6.78 ± 0.09 | 33.6 | 0.000 | 3.19–3.64 | ||
| G3 | 0.25 | 5.3 ± 1.2 | 0.19 ± 0.2 | 6.8 ± 0.12 | 33.4 | 0.000 | 3.17–3.62 | ||
| G4 | 0.5 | 5.2 ± 1.4 | 0.29 ± 0.05 | 10.19 ± 0.03 | 0.19 | 0.999 | −0.21–0.24 | ||
| G5 | 1 | 3.7 ± 0.6 | 0.17 ± 0.16 | 6.8 ± 0.12 | 33.4 | 0.000 | 3.17–3.62 | ||
| G6 | Normal Saline | 3.2 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100 | 0.000 | 9.97–10.4 | ||
One-way ANOVA was performed for the ulcer index. ∗∗ Post hoc analysis was performed at the 0.05 significance level; comparisons were performed between G1 (the negative control) and the test groups; bold numbers represent significant results at P0.05.
4. Discussion
An efficient method for the isolation of proteins, particularly lectins, usually entails the use of an extraction buffer to increase the lectin yield while minimizing contamination. It is common practice to opt for a buffer with a pH close to neutral, as purified lectins often demonstrate peak activity under such conditions. Given that most proteins have isoelectric points (pIs) between 4 and 6 and precipitate, leading to permanent inactivation, extraction buffers with low pH values are typically avoided in protein isolation processes [26,43]. Low pH buffers can adversely affect the stability and activity of certain lectins by disrupting their native structure and functional integrity. This disruption can lead to unfolding or denaturation of the lectin protein, impairing its ability to maintain proper tertiary and quaternary structures necessary for biological activity. Additionally, low pH conditions may alter the lectin's binding affinity to its ligands, thereby diminishing its effectiveness in biological processes such as carbohydrate recognition or cell surface interactions [44]. Hence, among the various buffers initially used for lectin extraction, PBS proved to be the most effective in extracting the majority of lectin. Like many other plant lectins, the crude protein fractions obtained from both the seeds and leaves of T. brownii contain a combination of closely related isolectins, which were isolated from the protein fractions precipitated by ammonium sulfate. These isolectins generally have the potential to display diverse carbohydrate specificities, as well as varying hemoagglutinating activities across different blood groups and types. However, unlike the isolectins identified in the TerBL and TerBS fractions, which consistently behaved similarly toward all tested simple carbohydrates, none of these sugars could inhibit the hemagglutinating activities of these isolectins. Additionally, isolectins exhibit distinct specificities towards erythrocytes from various sources [29,45]. The purified isolectins from Phaseolus vulgaris (L4, L3E1, L2E2, L1E3, and E4) exhibited significant variations, particularly with E4, which displayed no affinity for any of the tested human erythrocytes. Researchers have frequently focused on the number of subunits per isolectin, with a higher subunit count per molecule suggesting stronger binding of the lectin to the erythrocyte receptor [46]. The diverse forms of plant lectins underscore the varied biological significance inherent within these proteins. This diversity in structure and function highlights the intricate roles that plant lectins play in various biological processes and pathways [25]. Concerning their sugar-binding characteristics, plant lectins are typically categorized into three groups: mannose-specific lectins, galactose/galactosamine-specific lectins, and fucose-specific lectins [47]. From a physiological standpoint, plant lectins often exhibit limited recognition of simple sugars. Their biological interactions with simple sugars are recurrently uncommon or infrequent [48]. Numerous studies have proposed that plant lectins tend to favour binding to complex glycan structures over simple sugars [49,50]. Given that none of the tested simple sugars inhibited the hemagglutinating activity of the lectin, utilizing the glycoprotein fetuin as an affinity matrix emerged as a fitting choice. Moreover, the restoration of the loss of activity of TerBLL upon the addition of equimolar metal ions (Mn+2 and Ca+2) following elution with 3 % acetic acid confirms the involvement of metal ions in the carbohydrate-binding domain of the lectin [[51], [52], [53]]. The notable increase in specific activity observed in TerBLL and TerBSL following the fetuin affinity purification step indicates both the suitable choice of fetuin as an affinity matrix and the effectiveness of the purification procedure in enhancing the potency of the lectins per protein unit. The purification fold for TerBLL and TerBSL increased by 5.5 and 4.5, respectively, showing the significant enrichment attained through the purification process in comparison to that of the crude sample.
Plant lectins exhibit remarkable stability, enduring under a variety of harsh physiochemical conditions, including extreme pH [54,55], harsh temperatures, and various concentrations of potent denaturing agents [29,[56], [57], [58]]. Despite these challenges, plant lectins can retain some residual activity. Urea is commonly employed as a denaturing agent for proteins due to its impact on both hydrogen and hydrophobic bonds [59]. Our findings with TerBLL, which shows high resistance to urea, suggest that hydrophobic interactions might not be crucial for maintaining lectin subunits or the orientation of the lectin carbohydrate binding site, excluding such bonds [60]. Despite the significant variability in various physicochemical characteristics between TerBLL and TerBSL, this study indicated that they possess similar architectures in terms of subunit molecular weights. The prevalence of plant lectins with uneven molecular weights is widespread across the plant kingdom [[61], [62], [63], [64]], which is believed to grant them the variable ability to recognize different sugars or glycans [65]. Lectins derived from both seeds and other vegetative tissues of the same plant can be controlled by different genetic codes [66]. Plants usually produce a variety of lectins that serve specific roles determined by their location and biological function within the plant. These lectins may have distinct genetic origins and structural characteristics that enable them to function effectively in various tissues and environmental conditions [67]. Therefore, while there may be similarities, lectins from seeds and leaves can be encoded by distinct genes to meet the specialized needs of these plant parts. In terms of molecular subunit structure, the plant lectin from this species, along with its counterpart from Combretum glutinosum seeds [21], exhibits not only a nearly identical number of subunits but also similar molecular weights. Both lectins from these different plants are heterodimeric, with subunit molecular weights of approximately 50 and 60 kDa.
Pain is simply defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [68]. Throughout history, medicinal plants have continuously demonstrated their immense value as a rich source of numerous molecules with therapeutic importance. Even today, they remain an indispensable reservoir for the exploration and discovery of novel pharmaceutical agents, offering promising avenues for the development of new drugs. Due to their unique carbohydrate recognition features and abundance, plant lectins have been routinely used in many biological frontiers for protein-carbohydrate recognition, such as detection, purification and detailed studies of glycoconjugates, histochemistry of cells and tissues, and cancer cell identification [68].
Plant lectins have been investigated for their potential analgesic effects, yet the precise mechanisms driving their analgesic effects remain incompletely elucidated. Nonetheless, they are believed to exert their antinociceptive effects through the following pathways:
-
1.
Interaction with Nociceptive Pathways: Lectins may interact with nociceptive pathways in the body, modulating the transmission of pain signals from peripheral nerves to the central nervous system. By interfering with the activation of pain receptors or neurotransmitter release, lectins may reduce the perception of pain.
-
2.
Inhibition of Inflammatory Mediators: Many plant lectins have been found to possess anti-inflammatory properties. By inhibiting the release or activity of inflammatory mediators such as prostaglandins, cytokines, and histamines, lectins can help reduce inflammation-associated pain [20].
-
3.
Modulation of Ion Channels: Lectins may modulate the activity of ion channels involved in pain signalling, such as voltage-gated sodium channels and transient receptor potential (TRP) channels. By altering the excitability of sensory neurons, lectins can affect the transmission of pain signals [69].
-
4.
Interactions with Opioid Receptors: Certain plant lectins may interact with opioid receptors in the central nervous system, similar to endogenous opioid peptides. By binding to opioid receptors, lectins can activate analgesic pathways and reduce pain sensation [70].
The mechanism through which TerBLL alleviates pain in the present study remains to be fully comprehended. However, there is a suspicion that it may inhibit nociception induced by acetic acid, either by acting peripherally and centrally or solely peripherally. The induction of pain through the intraperitoneal injection of acetic acid occurs via the activation of chemosensitive nociceptors or the irritation of visceral surfaces. This irritation results in the release of histamine, bradykinin, prostaglandins, and serotonin. These mediators then activate chemosensitive nociceptors, contributing to the development of inflammatory pain. Therefore, TerBLL is likely to alleviate acetic acid-triggered pain by inhibiting the release of these mediators induced by chemical irritants [71]. Our findings indicate that TerBLL also alleviates pain by targeting the same receptors as morphine. Morphine is typically administered alongside its antagonist naloxone. Contemporary pain relievers such as morphine primarily alleviate pain by binding predominantly to the μ-opioid receptor, although they also show affinity for kappa and delta opioid receptors [72,73]. Similarly, among the various opioid receptor subtypes in the central nervous system (CNS), naloxone has the highest affinity for mu-opioid receptors [74]. These compounds swiftly counteract the effects of morphine, heroin, and oxycodone by acting as antagonists [75]. Thus, since naloxone nullifies the analgesic effect of TerBLL, the observed analgesic properties of TerBLL likely involve the μ-opioid receptor.
The quest for a plant protein to act as an antiulcer agent is captivating for its potential to harness nature's therapeutic bounty. Plants have long been known for their medicinal properties, and exploring their diverse proteins holds promise for identifying novel treatments. Plant proteins exhibit unique interactions with biological systems, suggesting enhanced compatibility and minimal side effects. Moreover, their natural origin aligns with the growing interest in eco-friendly healthcare solutions. Discovering a plant protein with antiulcer activity could revolutionize ulcer treatment, offering effective, sustainable, and environmentally conscious therapies [76]. TerBLL exhibited a gastroprotective effect comparable to that of pantoprazole, a proton pump inhibitor (PPI) medication prescribed for peptic ulcers due to its effective reduction in stomach acid production, a significant factor in the development and worsening of peptic ulcers [77]. Alcohol concentrations below 5 % typically stimulate acid secretion, leading to the generation of reactive oxygen species (ROS) that initiate lipid peroxidation in gastric tissues, ultimately resulting in significant damage to the stomach mucosal layers [78]. Conversely, alcohol concentrations exceeding 40 % are known to induce the secretion of gastric bicarbonate by promoting intercellular leakage. This process is believed to function as an intrinsic defense mechanism mediated through Cl2/HCO3 exchangers aimed at counteracting the effects of increased hydrochloric acid levels in the stomach [79]. Based on our current findings, although the highest dose of lectin (1 mg/kg) significantly protected the stomach, maintaining gastric pH levels near the negative control's baseline, it seems that the increase in pH observed at the lowest lectin dose (0.25 mg/kg), which also demonstrated significant protection, is likely due to ethanol rather than the lectin's action. These results suggest that TerBLL exerts its gastric protective effects through a mechanism other than elevating gastric pH, which warrants further investigation. To the best of our knowledge, this is the second report of a lectin from the Combretaceae family, both of which, when purified, demonstrate gastroprotective effects in experimental animals [21]. This finding underscores the traditional use of plants from this family to alleviate ulcer pain [80]. In a comprehensive review led by Sharifi-Rad and colleagues (Sharifi-Rad et al., 2018), a thorough analysis was conducted on aqueous and organic extracts sourced from various parts of plants recognized for their antiulcer properties, including the bark, leaves, and fruits. The authors also underscored several Terminalia species (Terminalia catappa L., Terminalia coriacea, Terminalia arjuna, Terminalia belerica, Terminalia chebula, and Terminalia fagifolia) for their considerable potential as gastroprotective agents. Although all of these plant extracts demonstrated gastroprotective effects, such as lowering gastric pH, reducing volume, or strengthening the mucosal lining, none of the studies succeeded in isolating active components from the crude extracts used. Notably, these studies utilized crude extracts in substantial quantities, ranging from 100 mg/kg to 500 mg/kg, to achieve a demonstrable antiulcer effect [76]. In contrast, our methodology incorporated the use of pure protein at a maximum dose of 0.25 mg/kg, proving to be adequately effective in eliciting substantial gastroprotection. Therefore, our study, grounded in our understanding and evidence, marks the initial endeavor to link the previously noted gastroprotective effects in Terminalia species, potentially to lectins.
5. Conclusion and future research perspectives
In summary, this study focused on the isolation, purification, and characterization of two lectins, TerBLL and TerBSL, from the leaves and seeds of Terminalia brownii Fresen. The extraction of crude proteins revealed that PBS was the most suitable buffer for lectin extraction based on lectin activity despite its lower protein concentration. The purification process using fetuin-agarose affinity chromatography resulted in significant enrichment of the lectin content, demonstrating the effectiveness of the chosen method. Both lectins displayed diverse hemagglutination activities in human and animal blood cells, indicating multiple potential isoforms. SDS‒PAGE analysis revealed subunit molecular weights of 57.3 kDa and 65.7 kDa for both TerBLL and TerBSL, respectively. Metal ion dependence studies indicated that both lectins are metalloproteins, and the use of divalent metal ions could restore their hemagglutinating activities. The lectins exhibited remarkable stability under various conditions, including high temperature, extreme pH, and urea treatment. Tryptophan modification studies and stability assessments under denaturing conditions provided insights into the structural characteristics of the lectins. Interestingly, TerBLL demonstrated an analgesic effect, alleviating thermal and heat-induced pain, possibly through blocking opioid receptors. These findings suggest that TerBLL has potential as a candidate for future analgesic drug development. This study also explored the antinociceptive effects of TerBLL using the writhing test, which revealed a significant reduction in abdominal writhing at higher doses. In the hotplate test, TerBLL demonstrated an analgesic effect comparable to that of morphine, with the involvement of the opioid pathway, as evidenced by the ability of naloxone to reverse the analgesic effects. Additionally, TerBLL exhibited significant gastroprotective properties against ethanol-induced ulcers, possibly through mechanisms other than increasing the pH at higher concentrations. As ulcer medications primarily target acid secretion inhibition, neutralization, or enhancement of the stomach mucosa lining, the latter option becomes more probable. In general, this research illuminates the possible biological importance of TerBLL and TerBSL. These findings underscore their varied activities, stability, analgesic and gastroprotective properties, positioning them as promising subjects for additional investigation and potential therapeutic uses. Given that certain species, including those in the present study, within the Combrataceae family are recognized as food sources [81] and that some have been proposed as novel food options [82]. It is important to note that the therapeutic applications of these lectins in current treatments and therapies remain speculative. Further research is required to validate their efficacy and safety in clinical settings, particularly in investigating the molecular mechanisms of lectin action in detail and exploring other biological activities. This will help address the potential limitations in translating these findings into practical therapeutic interventions. Future research should focus on detailed mechanistic studies and clinical trials to explore the full therapeutic potential of TerBLL and TerBSL, especially in their applications for pain management and gastrointestinal protection.
Ethical considerations for human and animal subjects
This research protocol was rigorously reviewed and approved by the Africa City of Technology Ethical Review Committee (Approval No: Eth-App-Biotech/12–2019). All blood donor participants were thoroughly informed about the aims, objectives, and potential implications of the project. Prior to their participation, each donor provided informed verbal consent, confirming their understanding and voluntary involvement. Informed consent was also obtained from participants (or their legal guardians) for the publication of any images, clinical data, and other data included in the manuscript.
Blood samples were collected exclusively for hemagglutination tests aimed at evaluating lectin activity and stability, adhering strictly to scientific and ethical standards. Additionally, animal experiments were conducted in accordance with the highest ethical standards for the humane treatment of animals, ensuring their welfare throughout the study. The research complies with all relevant national and international ethical guidelines for both human and animal subjects (Supplementary file S1).
CRediT authorship contribution statement
Ahmed H. Idries: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Eva H. Naser: Validation, Methodology, Investigation, Formal analysis. Maha B. Dafalla: Validation, Methodology, Investigation, Formal analysis. Sara A.A. Elmubarak: Validation, Methodology, Investigation, Formal analysis. Yusria E. Abdelrahim: Methodology, Investigation. Entsar A. Abdalrhman: Methodology, Investigation. Sabri Mustafa Alwali: Methodology. Bashir M. Ahmed: Methodology. Bashir A. Yousef: Writing – review & editing, Writing – original draft, Formal analysis. Reem M.A. Ebrahim: Methodology. Ashraf O. Abdellatif: Formal analysis. Amna K.E. Awadallah: Methodology. Makarim Elfadil M. Osman: Writing – review & editing, Writing – original draft, Visualization, Validation, Formal analysis. Emadeldin H.E. Konozy: Writing – review & editing, Writing – original draft, Project administration, Conceptualization.
Data availability
All the data utilized in this work are presented within the manuscript.
Fund
No funds were received for performing this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the deans of the Faculty of Veterinary Medicine at both the University of Bahri and the University of Khartoum for generously supplying the animal blood samples. Additionally, the latter institution graciously permitted the housing of the animals at their facility and facilitated the performance of certain experiments there.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39351.
Contributor Information
Ahmed H. Idries, Email: drahmeduofk@gmail.com.
Eva H. Naser, Email: evonhayder@gmail.com.
Maha B. Dafalla, Email: mbdaf@yahoo.com.
Sara A.A. Elmubarak, Email: elmubarksara@gmail.com.
Yusria E. Abdelrahim, Email: yusriaelshekh@gmail.com.
Entsar A. Abdalrhman, Email: eintisarbdalnabi22@gmail.com.
Sabri Mustafa Alwali, Email: sabrimustafa32@gmail.com.
Bashir M. Ahmed, Email: Beltegani@gmail.com.
Bashir A. Yousef, Email: bashiralsiddiq@gmail.com.
Reem M.A. Ebrahim, Email: reemaaboalsoud@gmail.com.
Ashraf O. Abdellatif, Email: ashrafpharm11@gmail.com.
Amna K.E. Awadallah, Email: amkh_elhaj@yahoo.com.
Makarim Elfadil M. Osman, Email: makarim84@gmail.com.
Emadeldin H.E. Konozy, Email: ehkonozy@yahoo.com, ehkonozy@ucc.edu.gh.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Mishra A., Behura A., Mawatwal S., Kumar A., Naik L., Mohanty S.S., Manna D., Dokania P., Mishra A., Patra S.K., Dhiman R. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019;134 doi: 10.1016/j.fct.2019.110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharon N., Lis H. Lectins--proteins with a sweet tooth: functions in cell recognition. Essays Biochem. 1995;30:59–75. [PubMed] [Google Scholar]
- 3.Osman M.E.M., Konozy E.H.E. Insight into Erythrina Lectins: properties, structure and proposed physiological significance. Open Bioact. Compd. J. 2017;5(1) [Google Scholar]
- 4.Kremsreiter S.M., Kroell A.H. Glycan-lectin interactions in cancer and viral infections and how to disrupt them. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konozy E., Osman M., Dirar A. Plant lectins as potent Anti-coronaviruses, Anti-inflammatory, antinociceptive and antiulcer agents. Saudi J. Biol. Sci. 2022;29(6) doi: 10.1016/j.sjbs.2022.103301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sousa G.F., Lund R.G. The role of plant lectins in the cellular and molecular processes of skin wound repair: an overview. Curr. Pharmaceut. Des. 2023;29(33):2618–2625. doi: 10.2174/0113816128264103231030093124. [DOI] [PubMed] [Google Scholar]
- 7.Al-Thobaiti S.A., Konozy E.H.E. Purification, partial characterization, and evaluation of the antiulcer activity of calotropis procera leaf lectin. Protein Pept. Lett. 2022;29(9):775–787. doi: 10.2174/0929866529666220803162457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan F., Khan R.H., Sherwani A., Mohmood S., Azfer M.A. Lectins as markers for blood grouping. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2002;8(12):RA293–300. [PubMed] [Google Scholar]
- 9.Melnykova N.M., Mykhalkiv L.M., Mamenko P.M., Kots S.Y. Вiopolymers and Cell. 2013. The areas of application for plant lectins. [Google Scholar]
- 10.Konozy E.H.E., Osman M.E.-f.M. Plant lectin: a promising future anti-tumor drug. Biochimie. 2022;202:136–145. doi: 10.1016/j.biochi.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kimutai M.B., Were M.L., Ambrose K.K., Mibey R. Extraction and analysis of spectral properties and ChroMophoric characterization of natural dye extract from barks of Terminalia brownii fresen (Combretaceae) Am. J. Appl. Chem. 2019;7(4) 166–122. [Google Scholar]
- 12.Salih E.Y., Fyhrquist P., Abdalla A.M., Abdelgadir A.Y., Kanninen M., Sipi M., Luukkanen O., Fahmi M.K., Elamin M.H., Ali H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen) Antibiotics. 2017;6(4):37. doi: 10.3390/antibiotics6040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kipkore W., Wanjohi B., Rono H., Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomed. 2014;10(1):1–22. doi: 10.1186/1746-4269-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wambugu S.N., Mathiu P.M., Gakuya D.W., Kanui T.I., Kabasa J.D., Kiama S.G. Medicinal plants used in the management of chronic joint pains in Machakos and Makueni counties, Kenya. J. Ethnopharmacol. 2011;137(2):945–955. doi: 10.1016/j.jep.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Kaigongi M., Musila F. Ethnobotanical study of medicinal plants used by Tharaka people of Kenya. J. Ethnobiol. Ethnomed. 2015;1(1):1–8. [Google Scholar]
- 16.Mbwambo Z.H., Moshi M.J., Masimba P.J., Kapingu M.C., Nondo R.S. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Compl. Alternative Med. 2007;7:1–5. doi: 10.1186/1472-6882-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mbiri J.W., Ogila K., Kisangau P., Gicheru M. Terminalia brownii fresen: stem bark dichloromethane extract alleviates pyrogallol-induced suppression of innate immune responses in Swiss albino mice. Evid. base Compl. Alternative Med. 2023;2023 doi: 10.1155/2023/9293335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardanyan R., Hruby V. Synthesis of essential drugs. 1stEd. Elsevier; 2006. ISBN: 9780080462127. [Google Scholar]
- 19.Singh A.K., Kumar S., Vinayak M. Recent development in antihyperalgesic effect of phytochemicals: anti-inflammatory and neuro-modulatory actions. Inflamm. Res. 2018;67:633–654. doi: 10.1007/s00011-018-1156-5. [DOI] [PubMed] [Google Scholar]
- 20.Oladokun B.O., Omisore O.N., Osukoya O.A., Kuku A. Anti-nociceptive and anti-inflammatory activities of Tetracarpidium conophorum seed lectin. Scientific African. 2019;3 [Google Scholar]
- 21.Naser E.H., Idries A.H., Elmubarak S.A.A., Dafalla M.B., Abdelrahim Y.E., Abdalrhman E.A., Ahmed B.M., Osman M.E.M., Awadallah A.K.E., Ebrahim R.M.A., Abdellatif A.O., Saad H.A., Konozy E.H.E. Isolation, purification, and characterization of lectins from medicinal plant Combretum glutinosum seeds endowed with analgesic and antiulcer properties. Biochimie. 2024 doi: 10.1016/j.biochi.2024.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Smith P.e., Krohn R.I., Hermanson G., Mallia A., Gartner F., Provenzano M., Fujimoto E., Goeke N., Olson B., Klenk D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Englard S., Seifter S. Precipitation techniques. Methods in enzymology, Elsevier. 1990:285–300. doi: 10.1016/0076-6879(90)82024-v. [DOI] [PubMed] [Google Scholar]
- 25.Awadallah A.K., Osman M.E.M., Ibrahim M.A., Bernardes E.S., Dias‐Baruffi M., Konozy E.H.E. Isolation and partial characterization of 3 nontoxic d‐galactose–specific isolectins from seeds of Momordica balsamina. J. Mol. Recogn. 2017;30(2):e2582. doi: 10.1002/jmr.2582. [DOI] [PubMed] [Google Scholar]
- 26.Konozy E.H., Bernardes E.S., Rosa C., Faca V., Greene L.J., Ward R.J. Isolation, purification, and physicochemical characterization of a D-galactose-binding lectin from seeds of Erythrina speciosa. Arch. Biochem. Biophys. 2003;410(2):222–229. doi: 10.1016/s0003-9861(02)00695-1. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.DuBois M., Gilles K.A., Hamilton J.K., Rebers P.t., Smith F. Colorimetric method for determination of sugars and related substances. Analytical chemistry. 1956;28(3):350–356. [Google Scholar]
- 29.Osman M.E.M., Awadallah A., Konozy E.H.E. Isolation purification and partial characterization of three lectins from Tamarindus indica seeds with a novel sugar specificity. Int. J. Plant Res. 2016;6(1):13–19. [Google Scholar]
- 30.Zhang W., Tian G., Geng X., Zhao Y., Ng T.B., Zhao L., Wang H. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules. 2014;19(12):19880–19891. doi: 10.3390/molecules191219880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spande T.F., Witkop B. Academic Press; 1967. Determination of the Tryptophan Content of Proteins with N-Bromosuccinimide, Methods in Enzymology; pp. 498–506. [Google Scholar]
- 32.I.o.L.A.R.C.o. Care. U.o.L. Animals . US Department of Health and Human Services, Public Health Service; 1986. Guide for the Care and Use of Laboratory Animals. National. [Google Scholar]
- 33.Koster R. Acetic acid for analgesics screening. Fed. Proc. 1959:412–417. [Google Scholar]
- 34.MacDonald A., Woolfe G., Bergel F., Morrison A., Rinderknecht H. Analgesic action of pethidine derivatives and related compounds. Br. J. Pharmacol. Chemother. 1946;1(1):4. [PMC free article] [PubMed] [Google Scholar]
- 35.Eddy N.B., Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J. Pharmacol. Exp. Therapeut. 1953;107(3):385–393. [PubMed] [Google Scholar]
- 36.Kulkarni S.K. 1987. Hand Book of Experimental Pharmacology, Vallabh Prakashan. [Google Scholar]
- 37.Dafalla M.B., Ebrahim R.M., Abdulaziz N.M., Elmubarak S.A., Naser E.H., Abdelrahim Y.E., Konozy E.H. Isolation, purification and characterization of a lectin from ocimum basilicum seeds (OBSL) with a complex sugar specificity. Neelain Journal of Science and Technology (NJST) 2022;6:1–10. [Google Scholar]
- 38.Singha B., Adhya M., Chatterjee B.P. Multivalent II [β-d-Galp-(1→ 4)-β-d-GlcpNAc] and Tα [β-d-Galp-(1→ 3)-α-d-GalpNAc] specific Moraceae family plant lectin from the seeds of Ficus bengalensis fruits. Carbohydr. Res. 2007;342(8):1034–1043. doi: 10.1016/j.carres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Khan M., Rahman A.M., Uddin M.S., Khatun S., Pervin F., Absar N. Purification and characterization of lectins from jute (Chorchorus olitorius) leaves. J. Chin. Chem. Soc. 2008;55(5):1171–1177. [Google Scholar]
- 40.Wang C., Eufemi M., Turano C., Giartosio A. Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry. 1996;35(23):7299–7307. doi: 10.1021/bi9517704. [DOI] [PubMed] [Google Scholar]
- 41.Halder S., Surolia A., Mukhopadhyay C. Impact of glycosylation on stability, structure and unfolding of soybean agglutinin (SBA): an insight from thermal perturbation molecular dynamics simulations. Glycoconj. J. 2015;32(6):371–384. doi: 10.1007/s10719-015-9601-y. [DOI] [PubMed] [Google Scholar]
- 42.Daniel V. In: Drug Discovery and Evaluation: Pharmacological Assays. Hock F.J., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. Peripheral analgesic activity; pp. 1–33. [Google Scholar]
- 43.Shen C.-H. In: Diagnostic Molecular Biology. second ed. Shen C.-H., editor. Academic Press; 2023. Chapter 8 - extraction and purification of proteins; pp. 209–229. [Google Scholar]
- 44.Singh R.S., Kaur H.P., Singh J. Purification and characterization of a mucin specific mycelial lectin from Aspergillus gorakhpurensis: application for mitogenic and antimicrobial activity. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panja A.S., Maiti S., Bandyopadhyay B. Protein stability governed by its structural plasticity is inferred by physicochemical factors and salt bridges. Sci. Rep. 2020;10(1):1822. doi: 10.1038/s41598-020-58825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egorin M.J., Bachur S., Felsted R.L., Leavitt R.D., Bachur N.R. Phaseolus vulgaris isolectin binding to human erythrocytes. J. Biol. Chem. 1979;254(3):894–898. [PubMed] [Google Scholar]
- 47.Wu A.M., Song S.-c., Tsai M.-s., Herp A. 2001. A Guide to the Carbohydrate Specificities of Applied Lectins-2, the Molecular Immunology of Complex Carbohydrates—2; pp. 551–585. [DOI] [PubMed] [Google Scholar]
- 48.De Schutter K., Van Damme E.J. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules. 2015;20(5):9029–9053. doi: 10.3390/molecules20059029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzmán-Partida A., Robles-Burgueno M., Ortega-Nieblas M., Vázquez-Moreno I. Purification and characterization of complex carbohydrate specific isolectins from wild legume seeds: Acacia constricta is (vinorama) highly homologous to Phaseolus vulgaris lectins. Biochimie. 2004;86(4–5):335–342. doi: 10.1016/j.biochi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Poiroux G., Barre A., Van Damme E.J., Benoist H., Rougé P. Plant lectins targeting O-glycans at the cell surface as tools for cancer diagnosis, prognosis and therapy. Int. J. Mol. Sci. 2017;18(6):1232. doi: 10.3390/ijms18061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Moreno M.R., Smith J.F., Smith R.V. Mechanism studies of coomassie blue and silver staining of proteins. J. Pharmaceut. Sci. 1986;75(9):907–911. doi: 10.1002/jps.2600750919. [DOI] [PubMed] [Google Scholar]
- 52.Brewer C.F., Marcus D.M., Grollman A.P., Sternlicht H. Interactions of saccharides with concanavalin A: relation between calcium IONS and the binding of saccharides to concanavalin a. J. Biol. Chem. 1974;249(14):4614–4616. [PubMed] [Google Scholar]
- 53.Brown R.D., III, Koenig S.H., Brewer C.F. Conformational equilibrium of demetalized concanavalin A. Biochemistry. 1982;21(3):465–469. doi: 10.1021/bi00532a008. [DOI] [PubMed] [Google Scholar]
- 54.Duk M., Lisowska E. Effect of pH on the binding of Vicia graminea lectin to erythrocytes: dependence on the chemical character of red‐cell receptors. Eur. J. Biochem. 1984;143(1):73–78. doi: 10.1111/j.1432-1033.1984.tb08342.x. [DOI] [PubMed] [Google Scholar]
- 55.Wongkham S., Wongkham C., Boonsiri P., Simasathiansophon S., Trisonthi C., Atisook K. Isolectins from seeds of Artocarpus lakoocha. Phytochemistry. 1995;40(5):1331–1334. doi: 10.1016/0031-9422(95)00535-f. [DOI] [PubMed] [Google Scholar]
- 56.Sathyapriya P., Arvinth S., Nadu T. Purification and properties of three novel monocot lectins from the family Zingiberaceae. International Journal of Advance Research, Ideas and Innovations in Technology. 2019;5(2):801–809. [Google Scholar]
- 57.Sage H.J., Vazquez J.J. Studies on a hemagglutinin from the mushroom Agaricus campestris. J. Biol. Chem. 1967;242(1):120–125. [PubMed] [Google Scholar]
- 58.Krishnaveni M., Kumar A.R., Ayyanar A. Isolation and characterization of mannose binding, thermostable lectin and its antibacterial activity against oral pathogens. Int. J. Multidiscip. Educ. Res. 2020;10(1):39–49. [Google Scholar]
- 59.Das A., Mukhopadhyay C. Urea-mediated protein denaturation: a consensus view. J. Phys. Chem. B. 2009;113(38):12816–12824. doi: 10.1021/jp906350s. [DOI] [PubMed] [Google Scholar]
- 60.Pando S., Macedo M., Freire M., Toyama M., Novello J., Marangoni S. Biochemical characterization of a lectin from Delonix regia seeds. J. Protein Chem. 2002;21:279–285. doi: 10.1023/a:1019797320348. [DOI] [PubMed] [Google Scholar]
- 61.Javed M., Bilal M., Tabassum B., Malik A., Adeyinka O.S., Tariq M., Nasir I.A. Purification and functional characterization of lectin from Chenopodium album. J. Protein Proteonomics. 2022;13(1):55–62. [Google Scholar]
- 62.Narahari A., Nareddy P.K., Swamy M.J. A new chitooligosaccharide specific lectin from snake gourd (Trichosanthes anguina) phloem exudate. Purification, physico-chemical characterization and thermodynamics of saccharide binding. Biochimie. 2011;93(10):1676–1684. doi: 10.1016/j.biochi.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 63.Suseelan K.N., Mitra R., Pandey R., Sainis K.B., Krishna T.G. Purification and characterization of a lectin from wild sunflower (Helianthus tuberosus L.) tubers. Arch. Biochem. Biophys. 2002;407(2):241–247. doi: 10.1016/s0003-9861(02)00517-9. [DOI] [PubMed] [Google Scholar]
- 64.Braga A.A., e Lacerda R.R., de Vasconcelos Medeiros G.K.V., Gonçalves G.F., de Luna Freire Pessoa H., Cardoso J.D., de Almeida Gadelha C.A., da Silva B.A., Santi-Gadelha T. Antibacterial and hemolytic activity of a new lectin purified from the seeds of Sterculia foetida L. Appl. Biochem. Biotechnol. 2015;175:1689–1699. doi: 10.1007/s12010-014-1390-4. [DOI] [PubMed] [Google Scholar]
- 65.Sinha S., Gupta G., Vijayan M., Surolia A. Subunit assembly of plant lectins. Curr. Opin. Struct. Biol. 2007;17(5):498–505. doi: 10.1016/j.sbi.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Van Damme E.J., Barre A., Rougé P., Van Leuven F., Peumans W.J. The seed lectins of black locust (Robinia pseudoacacia) are encoded by two genes which differ from the bark lectin genes. Plant Mol. Biol. 1995;29:1197–1210. doi: 10.1007/BF00020462. [DOI] [PubMed] [Google Scholar]
- 67.Naithani S., Komath S.S., Nonomura A., Govindjee G. Plant lectins and their many roles: carbohydrate-binding and beyond. J. Plant Physiol. 2021;266 doi: 10.1016/j.jplph.2021.153531. [DOI] [PubMed] [Google Scholar]
- 68.Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goyal S., Goyal S., Goins A.E., Alles S.R.A. Plant-derived natural products targeting ion channels for pain. Neurobiology of Pain. 2023;13 doi: 10.1016/j.ynpai.2023.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinho A.O., Brito J.S., da Costa J.A., da Silva A.R., da Silva S.P., de Amorim L.C., Correia M., Paiva P.M.G., de Oliveira A.M., Patriota L.L.S., Napoleão T.H. Schinus terebinthifolia leaf lectin has central and peripheral antinociceptive action mediated by its carbohydrate-recognition domain and delta-opioid receptors. J. Ethnopharmacol. 2023;301 doi: 10.1016/j.jep.2022.115817. [DOI] [PubMed] [Google Scholar]
- 71.Leite J.F.M., Assreuy A.M.S., Mota M.R.L., Bringel P.H.d.S.F., E Lacerda R.R., Gomes V.d.M., Cajazeiras J.B., Do Nascimento K.S., Pessôa H.d.L.F., Gadelha C.A.d.A., Delatorre P., Cavada B.S., Santi-Gadelha T. Antinociceptive and anti-inflammatory effects of a lectin-like substance from clitoria fairchildiana R. Howard seeds. Molecules. 2012;17(3):3277–3290. doi: 10.3390/molecules17033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L.-s., Wang J., Chen J.-c., Tao Y.-m., Wang Y.-h., Xu X.-j., Chen J., Xu Y.-g., Xi T., Hu X.-w. Novel κ-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation. Acta Pharmacol. Sin. 2015;36(5):565–571. doi: 10.1038/aps.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Listos J., Łupina M., Talarek S., Mazur A., Orzelska-Górka J., Kotlińska J. The mechanisms involved in morphine addiction: an overview. Int. J. Mol. Sci. 2019;20(17) doi: 10.3390/ijms20174302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansson J., Hirvonen J., Lovró Z., Ekblad L. Intranasal naloxone rapidly occupies brain mu-opioid receptors in human subjects. Neuropsychopharmacology. 2019;44(9):1667–1673. doi: 10.1038/s41386-019-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skolnick P. On the front lines of the opioid epidemic: rescue by naloxone. Eur. J. Pharmacol. 2018;835:147–153. doi: 10.1016/j.ejphar.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Sharifi-Rad M., Fokou P.V.T., Sharopov F. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23(7) doi: 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abed M.N., Alassaf F.A., Jasim M.H., Alfahad M., Qazzaz M.E. Comparison of antioxidant effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole. J. Pharmacol. 2020;105(11–12):645–651. doi: 10.1159/000506232. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y., Liang J., Wu J., Chen H., Zhang Z., Yang H., Chen L., Chen H., Su Z., Li Y. Transformation of patchouli alcohol to β-patchoulene by gastric juice: β-patchoulene is more effective in preventing ethanol-induced gastric injury. Sci. Rep. 2017;7(1):5591. doi: 10.1038/s41598-017-05996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chari S., Teyssen S., Singer M.J.G. Alcohol and gastric acid secretion in humans, BMJ-Journals. Gut. 1993;34(6):843. doi: 10.1136/gut.34.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emmanuel P.I., Sandra U.C., Marytheresa O.A., Nwakaego M.F., Nneoma O.M., Azubuike O.C., Uzor P.F. Gastroprotective effects of Combretum paniculatum (Combretaceae) leaf extract and fractions on absolute ethanol-induced gastric ulcer in rats. Future Journal of Pharmaceutical Sciences. 2022;8(1):54. [Google Scholar]
- 81.Das G., Kim D.Y., Fan C., Gutiérrez-Grijalva E.P., Heredia J.B., Nissapatorn V., Mitsuwan W., Pereira M.L., Nawaz M., Siyadatpanah A., Norouzi R., Sawicka B., Shin H.S., Patra J.K. Plants of the genus Terminalia: an insight on its biological potentials, pre-clinical and clinical studies. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.561248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weerawatanakorn M., Janporn S., Ho C.-T., Chavasit V. Terminalia catappa linn seeds as a new food source. Songklanakarin J. Sci. Technol. 2015;37:507–514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data utilized in this work are presented within the manuscript.