Abstract
Peripheral neuropathy is a common and debilitating complication of diabetes and prediabetes. Recent clinical studies have identified an association between the development of neuropathy and dyslipidemia in prediabetic and diabetic patients. Despite the prevalence of this complication, studies identifying molecular mechanisms that underlie neuropathy progression in prediabetes or diabetes are limited. However, dysfunctional mitochondrial pathways in hereditary neuropathy provide feasible molecular targets for assessing mitochondrial dysfunction in neuropathy associated with prediabetes or diabetes. Recent studies suggest that elevated levels of dietary saturated fatty acid (SFAs) associated with dyslipidemia impair mitochondrial dynamics in sensory neurons by inducing mitochondrial depolarization, compromising mitochondrial bioenergetics, and impairing axonal mitochondrial transport. This causes lower neuronal ATP and apoptosis. Conversely, monounsaturated fatty acids (MUFAs) restore nerve function and sensory mitochondrial function. Understanding the mitochondrial pathways that contribute to neuropathy progression in prediabetes and diabetes may provide therapeutic targets for the treatment of this debilitating complication.
Keywords: Diabetes, Prediabetes, Hereditary Neuropathy, Charcot-Marie-Tooth Disease, Mitochondria, Bioenergetics, Fusion, Fission, Mitochondrial associated membranes, Mitochondrial Trafficking
1. Introduction
The global burden of diabetes is a pressing health concern. Over 450 million people had diabetes in 2017, and it is estimated that numbers will surpass 700 million people by 2040. This is in addition to the staggering number of people with prediabetes, a condition characterized by impaired glucose tolerance that typically precedes overt type 2 diabetes (T2D); in the United States alone, roughly 34% of adults had prediabetes in 2015. Both diabetic and prediabetic individuals are prone to an array of complications, the most prevalent of which is neuropathy. Approximately 60% of individuals with T2D and 30% of individuals with prediabetes manifest with distal symmetric polyneuropathy (Callaghan, Price, & Feldman, 2015; Cortez, Singleton, & Smith, 2014; O’Brien, Hinder, Callaghan, & Feldman, 2017; Tabak, Herder, Rathmann, Brunner, & Kivimaki, 2012) and exhibit the typical “stocking and glove” pattern of progressive peripheral sensory nerve loss characterized by pain, numbness, eventual loss of sensation, and increased risk for amputation, all of which lead to significant morbidity and socioeconomic burden. In 2017, the estimated costs of diabetes totaled $327 billion USD, with $90 billion attributed to reduced productivity. This is further compounded by the lack of treatment options for neuropathy associated with diabetes and prediabetes, as the majority of management rests on lifestyle changes (Pop-Busui et al., 2017). Consequently, current research efforts are focused on identifying the biological pathways that underlie the onset and progression of neuropathy with the goal to develop novel therapeutic strategies and improve diagnostic approaches (O’Brien et al., 2017; O’Brien, Hinder, Sakowski, & Feldman, 2014; Vas & Edmonds, 2016; Zochodne, 2014).
Diabetic and prediabetic neuropathy are secondary to a series of metabolic perturbations that link hyperglycemia, dyslipidemia, and insulin resistance with inflammation, oxidative stress, and changes in gene expression. One unifying theme underlying these perturbations is mitochondrial dysfunction, the focus of this chapter. Neurons are reliant on a continuous supply of energy and are especially susceptible to mitochondrial impairment, and conditions that impede normal mitochondrial dynamics and function can lead to axonal dysfunction, sensory neuron injury and death, and ultimately to neuropathy. Here we build on these findings. In particular, we discuss relevant insights pertaining to mitochondrial dynamics and function during normal and diseased states, with particular emphasis on mitochondrial bioenergetics, mitochondrial morphology (including fission and fusion), endoplasmic reticulum (ER)-mitochondria interactions, and mitochondrial trafficking. We then concentrate on the most recent advances in our understanding of how metabolic imbalances common in diabetes and prediabetes, specifically hyperglycemia and dyslipidemia, affect mitochondrial dynamics in the peripheral nervous system (PNS).
2. Mitochondrial dynamics
2.1. Neurons rely on mitochondrial dynamics
Neurons, including sensory PNS neurons, are especially dependent on efficient mitochondrial dynamics to fulfill their energy needs due to constraints from their unique morphology (Pareyson, Saveri, Sagnelli, & Piscosquito, 2015). PNS neuron axons can measure 3 feet in length or longer and transmit signals from the extremities to the spinal cord, and their axon length versus cell body size ratio can be in excess of 20,000 (Feldman, Nave, Jensen, & Bennett, 2017; Wang, Medress, & Barres, 2012). Therefore, they need to distribute mitochondria over large distances from their cell body along axons to their terminal(s). Furthermore, neurons require massive amounts of energy to sustain their activity; neuronal survival requires a constant adenosine triphosphate (ATP) supply (Nicholls & Budd, 2000) and a single cortical neuron utilizes approximately 4.7 billion ATPs per second in the resting human brain (X. H. Zhu et al., 2012). The reliance of neurons on ATP for survival is highlighted by their mitochondrial density, which is greater in comparison to other cell types (Yu & Pekkurnaz, 2018). Additionally, neurons’ have energy demands for diverse tasks, and among the most consuming in brain neurons are housekeeping (~25% of energy), maintenance of resting membrane potential (~15%), firing of action potentials (~16%), and synaptic transmission (~44%) (Harris, Jolivet, & Attwell, 2012). Thus, their energy needs also are highly compartmentalized and neurons need to be able to direct mitochondria to the areas of highest energy consumption, such as at their synapses (Rossi & Pekkurnaz, 2019).
The constant need for ATP to survive means that neurons, including PNS neurons, critically rely on strictly controlled and coordinated mitochondrial dynamics to ensure sustained ATP production throughout their axons and synaptic terminal(s) (Sajic, 2014). Mitochondrial dynamics comprises several aspects of mitochondria, including fission/fusion (Section 2.3), mitophagy, and transport (i.e., trafficking, Section 2.5). Mitochondrial elongation, reshaping, and/or fusion is enhanced under conditions of nutrient starvation to increase the efficiency of ATP production and continue to meet the neuron’s energy demands (Yu & Pekkurnaz, 2018). Conversely, mitochondrial fission intensifies in response to increased nutrient supply and severe stress. For quality control, damaged mitochondria are cleared by mitophagy under conditions of elevated oxidative stress to enable replacement by properly functioning mitochondria. Overall, the relative extent of mitochondrial fission to fusion controls the range of mitochondrial size and morphology, which is also coordinated with mitophagy to optimize mitochondrial distribution and quality for optimal ATP production on which neurons rely.
In addition to regulation of mitochondrial turnover, neurons must ensure distribution of mitochondria from the cell body throughout the axon to guarantee they are delivered to sites in need of ATP (Pareyson et al., 2015). Thus, mitochondrial transport mechanisms must match ATP requirements within neuronal axons, otherwise loss of mitochondria results in insufficient ATP, impaired synaptic transmission, and axonal injury. Within the myelinated nerve, electrical activity regulates mitochondrial transport, suggesting that the axon’s demand for energy regulates mitochondrial trafficking (Ohno et al., 2011). The essential nature of proper mitochondrial trafficking in neurons is highlighted by loss or mutation to critical mitochondrial fission/fusion, and motor proteins, which leads to neurological pathologies, such as Charcot-Marie-Tooth disease (CMT, Section 3) and motor neuron diseases, the most common of which is amyotrophic lateral sclerosis (ALS) (Baloh, Schmidt, Pestronk, & Milbrandt, 2007; De Vos et al., 2007; De Vos & Hafezparast, 2017). In a similar vein, depletion of ATP, such as through metabolically-triggered mitochondrial dysfunction associated with diabetes and prediabetes (e.g., hyperglycemia and dyslipidemia, Sections 4 and 5) could have pathological consequences, leading to neuronal cell injury and neuropathy.
2.2. Mitochondrial bioenergetics
Mitochondria are the source of ATP production, the cell’s energy currency (Fig. 1). ATP is generated through a series of biochemical reactions, starting with the tricarboxylic acid cycle (TCA) (Krebs & Johnson, 1937) followed by oxidative phosphorylation (OXPHOS) (Chaban, Boekema, & Dudkina, 2014). The TCA cycle is fueled by acetyl-coenzyme A (acetyl-CoA), which can be synthesized from glycolysis-derived pyruvate or from fatty acid (FA) β-oxidation (Houten & Wanders, 2010). The TCA cycle generates reduced nicotinamide adenine dinucleotide (NADH) and reduced flavin adenine dinucleotide (FADH2), which drive OXPHOS and, ultimately, ATP production. Glycolytic metabolism within the cytoplasm rapidly releases 2 ATP molecules from catabolism of a single glucose molecule whereas oxygen-dependent energy conversion within mitochondria liberates, under ideal conditions, 36 additional ATP molecules by OXPHOS. The utilization of glycolysis versus OXPHOS during neuronal signal transmission is not precisely known, but evidence suggests that glycolysis serves as a readily available ATP source during periods of excessive energy demand, but that mitochondrial oxidative OXPHOS, which is more efficient, occurs during both basal conditions and periods of high neuronal activity (Rossi & Pekkurnaz, 2019).
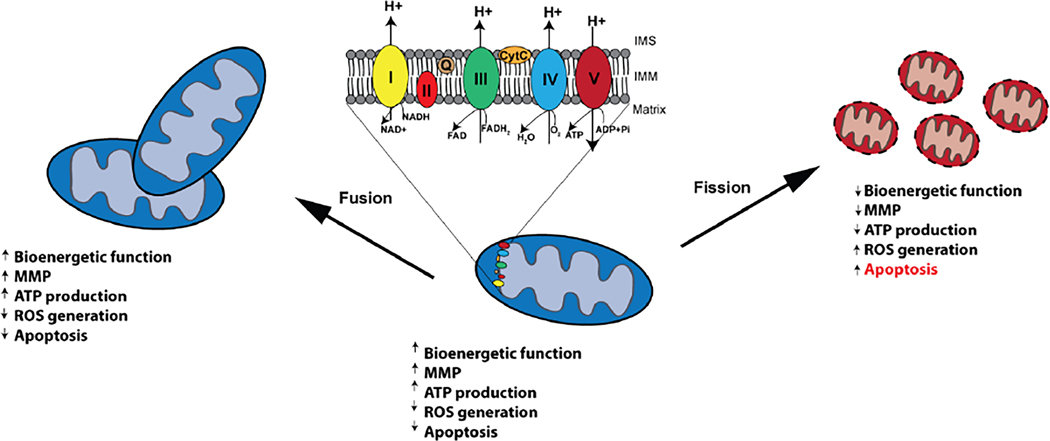
Fig. 1.
Schematic of mitochondrial fission and fusion dynamics. Mitochondrial bioenergetics are regulated by mitochondrial fission and fusion events. Mitochondrial fusion events retain mitochondrial bioenergetic function, MMP, ATP production, and decrease ROS generation and apoptosis. Conversely, mitochondrial fission leads to mitochondrial depolarization which impairs mitochondrial bioenergetic function and decreases ATP production. Compromised bioenergetics lead to ROS production triggering apoptosis.
Mitochondria are composed of an outer mitochondrial membrane (OMM) that surrounds an inner mitochondrial membrane (IMM), which folds inwardly forming cristae that expand the IMM surface area to maximize energy production. Within the cristae is the mitochondrial matrix, which is comprised of TCA components and other metabolites, lipids, ribosomes, transfer RNAs, and mitochondrial DNA, enzymes, and proteins, whereas OXPHOS enzymes reside within the IMM. The TCA cycle utilizes acetyl-CoA, which is broken down through a series of enzymatically catalyzed biochemical reactions to CO2, NADH, FADH2, guanosine triphosphate (GTP), and protons. Next, NADH and FADH2 serve as cofactors to complexes I and II, respectively, which are entry points into the OXPHOS electron transport chain (Cecchini, 2003; Hirst, 2005), and which are followed by complexes III and IV (Crofts, 2004; Tsukihara et al., 1996). Electrons move down the potential energy gradient along complexes of the electron transport chain, aided by shuttle proteins, which results in a net transport of protons from the matrix to the intermembrane space (IMS) (Chaban et al., 2014). This transfer of protons across the IMM generates a proton gradient that is dissipated through complex V (ATP synthase), which produces ATP as protons are transported from the IMS to the matrix (Boyer, 1997) (Fig. 1). The proton gradient also establishes the mitochondrial membrane potential (MMP), which is necessary for mitochondrial function, regulates the direction of mitochondrial transport (Miller & Sheetz, 2004), and prevents mitochondrial damage (Sivitz & Yorek, 2010).
In addition to the TCA cycle and OXPHOS, pyruvate produced by cytoplasmic glycolysis is shuttled into the mitochondrial matrix where a three-enzyme pyruvate dehydrogenase complex (PDC) converts it into acetyl-CoA through pyruvate decarboxylation (Patel, Nemeria, Furey, & Jordan, 2014). FA acyl-L-carnitine derivatives are also shuttled into mitochondria and catabolized by β-oxidation (Houten & Wanders, 2010; Kastaniotis et al., 2017). For even-chain FAs, a repeated 4-step cycle cleaves two carbon atoms at a time from the FA, which is converted into acetyl-CoA. For odd-chain FAs, the final cleavage yields propionyl-CoA in addition to acetyl-CoA.
Careful coordination of mitochondrial biochemical processes ensures efficient ATP generation. However, unanticipated side reactions or exogenous species can lead to suboptimal energy production or mitochondrial dysfunction, such as through the formation of reaction oxygen species (ROS) or toxic intermediates. ROS can be inadvertently produced at the electron transport chain by premature escape of a semi-reduced oxygen species, such as the superoxide anion (Angelova & Abramov, 2018). The MMP influences ROS production; in some contexts, a low MMP slows respiration, which increases ROS production, however, in certain instances, a high MMP triggers expression of uncoupling proteins that lower ROS but also drop ATP production. Thus, MMP and ROS are intimately linked and together impact mitochondrial efficiency, ATP production, and neuronal health.
Excessive influx of long-chain saturated fatty acids (LCSFAs) into mitochondria, for instance as occurs during dyslipidemia (Section 5), interferes with the normal physiological process of FA β-oxidation. When FA uptake overcomes a mitochondria’s capacity for FA β-oxidation, lipotoxic metabolic intermediates are generated, such as ceramides (Fucho, Casals, Serra, & Herrero, 2017), diacylglycerols, or cardiolipin (D’Souza, Nzirorera, & Kienesberger, 2016; DeFronzo, 2010), which negatively impact mitochondrial bioenergetics. Proteins involved in mitochondrial fission and fusion also influence mitochondrial size, and thus bioenergetic capacity. Conversely, the metabolic state (i.e., normal physiological or pathological conditions) affects mitochondrial fission and fusion (Section 2.3). Overall, mitochondrial bioenergetics and dynamics coordinate with the neuron’s energy demands to sustain ATP production and neuronal health. However, this carefully orchestrated process can be disrupted by aberrant metabolic states, including diabetes or prediabetes, or hereditary mutations, such as in CMT, ultimately leading to neuropathy.
2.3. Mitochondrial morphology, fission, and fusion
Within cells, and especially neurons that are particularly reliant on continuous ATP production, mitochondrial dynamics ensure that cellular energy demands are met. The balance between mitochondrial fission and fusion regulates their number, morphology, and size distribution, whereas trafficking delivers them to the locations they are needed (Fig. 1, Section 2.5). Mitochondria also coordinate with other cellular organelles, such as the ER, for lipid biosynthesis, calcium exchange, and signaling (Section 2.4).
The major players in mitochondrial fission include mitochondrial fission factor (MFF), mitochondrial fission 1 protein (FIS1), mitochondrial elongation factor 1 and 2 (MIEF1 and MIEF2), and ganglioside induced differentiation associated protein 1 (GDAP1) localized to the OMM and dynamin 1 like (DNM1L) in the cytoplasm (Bertholet et al., 2016). The precise mechanism for fission is not known, although an interaction of GDAP1 with DNM1L and FIS1 has been proposed (Niemann, Wagner, Ruegg, & Suter, 2009; Pareyson et al., 2015). DNM1L is recruited to mitochondria by its receptor proteins MFF and FIS1, or alternatively by MIEF1 and MIEF2, that can recruit DNM1L in the absence of MFF and FIS1 (Loson, Song, Chen, & Chan, 2013). DNM1L that is recruited to the OMM forms a ring-shaped oligomer around the mitochondria, which is also wrapped by ER (Friedman et al., 2011). In MIEF1- or MIEF2-mediated fission, the DNM1L ring oligomer dissociates from the MIEF1 or MIEF2 receptors and contracts to trigger fission (Kalia et al., 2018). DNM1L activity is regulated by several post-translational modifications (Chang & Blackstone, 2010), including O-GlcNAcylation. Changes in DNM1L activity can result in mitochondrial fragmentation and a lowering of MMP (Gawlowski et al., 2012). Similar loss of mitochondrial integrity and polarization can be triggered by hyperglycemia (Makino et al., 2011), such as occurs in diabetes.
Mitochondrial fusion is mediated by mitofusins 1 and 2 (MFN1 and MFN2), which are dynamin-related GTPases localized to the OMM, and optic atrophy 1 (OPA1), a mitochondrial dynamin-like GTPase localized to the IMM. MFN1 and MFN2 from two mitochondria form homo- and heterooligomers, that tether and initiate the fusing process of the OMM. Upon fusion, there is an interaction between MFN1 and OPA1 in the OMM that fuses the IMM (Meeusen et al., 2006; Meeusen, McCaffery, & Nunnari, 2004; Pareyson et al., 2015). The energy for the OMM fusion process derives from MFN1 and MFN2 GTPase activity whereas tethering is mediated by their C-terminal coiled-coil domain (Suen, Norris, & Youle, 2008). OPA1 is also the principle regulator of mitochondrial cristae organization, whose surface area impacts OXPHOS and therefore the efficiency of ATP production (Pernas & Scorrano, 2016). OPA1 deficiency can induce defects in mitochondrial morphology within optic nerves (Davies et al., 2007).
Neurofilaments (NFs) are cytoskeletal framework components that are specific to neurons (Gentil, Tibshirani, & Durham, 2015). They occur as light (NF-L), medium (NF-M), and heavy (NF-H) chains; the NF-H subunits run parallel to the axon, lending mechanical support, whereas the NF-M and NF-L run perpendicularly to the NF-H subunits (Pareyson et al., 2015; Sajic, 2014). NFs also organize organelles, in addition to mitochondria, whose length, rate of fusion, and motility is affected by NFs (Gentil et al., 2012).
Mutations to mitochondrial fission and fusion proteins GDAP1 and MFN2 and the gene encoding NF-L, neurofilament, light polypeptide (NEFL), and mitochondrial transport machinery (Section 2.5) are all associated with hereditary CMT neuropathy (Section 3) (Pareyson et al., 2015). Mutant OPA1 is linked to autosomal dominant optic atrophy, a heritable disease of optic nerve deterioration (Alexander et al., 2000; Delettre et al., 2000), whereas DNM1L mutations are connected with severe infantile encephalopathy disease. In addition to their more established roles in mitochondrial fission and fusion, mutations to MFN2 (Loiseau et al., 2007; Pich et al., 2005) and OPA1 (Chevrollier et al., 2008; Zanna et al., 2008) also lead to altered mitochondrial bioenergetics, MMP, and coupling defects (Fig. 1).
2.4. ER-mitochondrial interactions regulate mitochondrial dynamics
Mitochondria-associated ER membranes (MAMs) are juxtaposed interorganellar sites (Lopez-Crisosto et al., 2015). The ER and mitochondrial membranes are brought in very close proximity through bridging by various tethering complexes, but the membranes do not actually fuse (Csordas et al., 2006). Therefore, each organelle maintains its own identify, but the MAM contact sites enable mutual regulation of both organelles. MAM contact sites allow interorganellar transfer of calcium and lipids and control diverse cellular activities, ranging from mitochondrial dynamics, autophagy, and inflammation (Marchi, Patergnani, & Pinton, 2014).
Tethering proteins are located in the OMM and ER membranes and maintain MAMs stably or transiently. Four MAM tethering complexes have been identified in mammalian cells. MFN1 and MFN2 were originally discovered on the surface of mitochondria (Section 2.3), followed by discovery of MFN2 on ER membranes, where it helps form the MAM interface by homologous interaction with mitochondrial MFN2 or heterologous interaction with mitochondrial MFN1 (de Brito & Scorrano, 2008). This process is regulated by the mitochondrial ubiquitin ligase MITOL, which only modifies mitochondrial MFN2 at lysine 63 with a polyubiquitin chain that elicits oligomerization and subsequent tethering (Sugiura et al., 2013).
Another tethering complex is formed from the interaction between ER-localized vesicle associated membrane protein-associated protein B (VAPB) with mitochondria-localized protein tyrosine phosphatase-interacting protein 51 (PTPIP51) (De Vos et al., 2012). Voltage-dependent anion channel 1 (VDAC1) complexes with two other proteins, subtype 3 of the 1,4,5-triphosphate receptor (IP3R3) and 75 kDa glucose regulated protein (GRP75), to form another tethering complex, which regulates calcium exchange between mitochondria and ER (Szabadkai et al., 2006). A complex of ER-localized B-cell receptor associated protein 31 (BAP31), FIS1, and phosphofurin acidic cluster sorting protein-2 (PACS-2) form the tethering structure that regulates apoptosis (Iwasawa, Mahul-Mellier, Datler, Pazarentzos, & Grimm, 2011; Simmen et al., 2005). Lastly, a suite of additional proteins also associates with MAMs, including proteins or enzymes that participate in mitochondrial dynamics, lipid synthesis, and autophagy regulation, as well as ER chaperones.
MAMs perform several key functions to maintain neuronal health. Calcium transport between ER and mitochondria is primarily mediated by the GRP75-IP3R3-VDAC1 tethering complex through interaction with sigma non-opioid intracellular receptor 1 (SIGMAR1), an ER chaperone protein. SIGMAR1 blocks breakdown of IP3R3 to sustain calcium transfer across MAMs, which is essential for ER and mitochondrial function (Hayashi, Rizzuto, Hajnoczky, & Su, 2009). MAMs can also regulate autophagosome formation through the tethering proteins MFN2 (Hamasaki et al., 2013), SIGMAR1 (Vollrath et al., 2014), and VAPB-PTPIP51 (Gomez-Suaga et al., 2017), and mitophagy through PTEN-induced kinase 1 (PINK1) and Beclin-1 (BECN1) (Gelmetti et al., 2017). Importantly for mitochondrial dynamics, MAMs impact mitochondrial morphology, transport, fission, and fusion (Bernard-Marissal, Chrast, & Schneider, 2018). ER and mitochondria mutually affect each other’s morphology through MAMs via MFN2. Loss of MFN2 triggers ER expansion and protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) activation, leading to aberrant mitochondrial morphology and function (Munoz et al., 2013). MAMs influence mitochondrial transport through the interaction of MFN2 with mitochondrial Rho GTPase 1 and 2 (Miro 1 and 2, Section 2.5), receptor proteins that tether mitochondria to motor proteins in a calcium-dependent fashion. MAMs also stimulate mitochondrial fission and fusion via the tethering proteins MFN1 and MFN2, as well as through MAM-associated DNM1L (Friedman et al., 2011) or inverted formin-2 (INF2) (Korobova, Ramabhadran, & Higgs, 2013).
Overall, tethering complexes and other MAM-associated proteins coordinate to regulate calcium, auto- and mitophagy, and mitochondrial transport and dynamics (fission, fusion). Aberrant mitochondrial and ER function is an early event in the course of neuronal degeneration; in parallel, deficiencies in MAM function similarly leads to neuronal injury (Bernard-Marissal et al., 2018). Mutation to some of these tethering and MAM-associated proteins results in CMT (Section 3.1.3) or to familial forms of ALS (Bernard-Marissal et al., 2018), underscoring the importance of MAMs to healthy neuronal function.
2.5. Mitochondrial trafficking and motor proteins
Mitochondria are transported along neuronal axons by the synchronized effort of numerous proteins involved in the motor machinery. Broadly speaking, the machinery is composed of the molecular motors that achieve step-wise movement along a microtubule track with receptor and adaptor proteins that secure the mitochondria cargo to the motors (Mishra & Chan, 2016). Mitochondria need to be transported along axons in either direction, so anterograde and retrograde transport are both essential. Kinesin-1 [KIF5A, KIF5B, KIF5C (Kawaguchi, 2013)] and dynein motor proteins conduct anterograde and retrograde mitochondrial transport, respectively (Saxton & Hollenbeck, 2012; Sheng, 2014). A canonical kinesin-1 motor is comprised of three principal domains, an ATPase motor domain that takes steps along microtubules, and a heavy and light chain, which join the motor domain to the adaptor proteins and cargo (Hirokawa & Takemura, 2005). The dynein motor is a multi-protein complex, which also contains an ATPase motor domain, in addition to heavy, intermediate, and light chain domains, and other components, including dynactin (DCTN), that join the motor domain to the adaptor proteins and mitochondrial cargo (Roberts, Kon, Knight, Sutoh, & Burgess, 2013; Sajic, 2014).
Securing the mitochondrial cargo to the motors are a number of receptor and adaptor proteins (Mishra & Chan, 2016). The main receptor proteins are Miro1 and Miro2, which are expressed on the outer mitochondrial membrane (Nguyen et al., 2014) and are required for mitochondrial transport (Fransson, Ruusala, & Aspenstrom, 2006). They possess calcium-binding binding EF-hand motifs (Lee & Lu, 2014), which are able to sense calcium levels, and regulate binding or release of mitochondria from the transport machinery. Trafficking kinesin-binding protein 1 and 2 (TRAK1 and TRAK2, also referred to as Milton) are adaptor proteins that interact with Miro and kinesins to fasten the mitochondria to the motor (Brickley & Stephenson, 2011) and are also essential for mitochondrial trafficking (van Spronsen et al., 2013). Syntaphilin is another protein that anchors mitochondria to microtubules in regions of high local calcium levels (Chen & Sheng, 2013; Kang et al., 2008).
The final component of the axonal transport machinery are the tracks themselves, the microtubules that run along axons. Microtubules form by the polymerization of α-tubulin and β-tubulin dimers (Conde & Caceres, 2009). Polymerization of the dimers instills an inherent polarity to the microtubule tracks, with a minus end on the α-tubulin side, which is usually directed towards the cell body, and a plus end on the β-tubulin side, which is distal and is generally directed towards the axon terminals. The polarity also defines the transport direction since kinesin prefers to move towards the plus end (anterograde) (Hirokawa et al., 1991) and dynein, although bidirectional (Ross, Wallace, Shuman, Goldman, & Holzbaur, 2006), favors motion towards the negative end (retrograde) (Pilling, Horiuchi, Lively, & Saxton, 2006). Movement in either direction is carefully regulated by a number of mechanisms (Nirschl, Ghiretti, & Holzbaur, 2016).
The concerted interaction of the mitochondria cargo, receptor and adaptor proteins, motors, and microtubule tracks ensures efficient mitochondrial trafficking. Loss of function mutations to mitochondrial trafficking proteins, such as KIF5A and TUBB3 (β-tubulin subtype) in hereditary CMT neuropathy (Section 3.1.4) (Pareyson et al., 2015). Under normal physiological conditions, a number of environmental cues, such as ATP, calcium, glucose, and oxygen levels, can regulate movement of mitochondria along axons or attachment/detachment of mitochondria to/from the motor tracks (Mishra & Chan, 2016; Saxton & Hollenbeck, 2012). Unsurprisingly, disease conditions, such as hyperglycemia (Section 4.4) or dyslipidemia (Section 5.5) in diabetic patients will disrupt regulatory mechanisms governing mitochondrial trafficking, leading to neuropathy.
3. Mitochondrial dynamics in disease
Understanding dysfunctional mitochondrial pathways associated with genetic neuropathic diseases, including hereditary neuropathies, has incredible potential to offer insight into molecular mechanisms of mitochondrial dysfunction that may be pertinent to neuropathy progression in prediabetes and diabetes. In this section, we will therefore discuss relevant advances in mitochondrial bioenergetics, mitochondrial fusion and fission, ER-mitochondrial interaction sites, and mitochondrial trafficking that have been identified through studies of mitochondrial pathways underlying other hereditary neuropathies (Table 1). These insights have paved the way for the recent progress and emerging research on mitochondrial dysfunction in neuropathy associated with diabetes and prediabetes that will be discussed in subsequent sections.
Table 1.
Mutations to genes involved in mitochondrial defects in CMT, other peripheral neuropathies, and optic atrophy.
| Gene (alphabetically) | Associated CMT neuropathy (OMIM#) | Mode of inheritance | Affected mitochondrial dynamics | Function |
|---|---|---|---|---|
| AIFM1 | CMTX4 (310490) | X-linked | Mitochondrial metabolism/apoptosis signaling | A FAD-dependent NADH oxidase with a C-terminal catalytic domain facing the IMS, cleaves under pro-apoptotic cues, moves to nucleus to initiate apoptosis |
| DHTKD1 | CMT2 (615025) | Autosomal dominant | Bioenergetics/mitochondrial metabolism | Encodes the E1 subunit of the mitochondrial amino acid degrading alpha-ketoadipic acid dehydrogenase complex |
| DNM1L | Severe infantile encephalopathy (614388) | AD, AR | Mitochondrial fission | Recruited to mitochondrial OMM by MFF, FIS1, MID49, or MID51 receptors, oligomerizes in a ring-like structure to trigger fission |
| DYNC1H1 | CMT2O (614228) SMALED (158600) |
Autosomal dominant | Mitochondrial trafficking | Subunit of dynein motor protein that performs retrograde axonal transport of mitochondria |
| GDAP1 | CMT4A (214400) CMT2K (607831) CMT2K (607831) CMTRIA (608340) |
AR AR AD AR |
Mitochondrial fission | Forms complexes with DRP1 and FIS1 to effect mitochondrial fission |
| HK1 | CMT4G or HMSN Russe (605285) | Autosomal recessive | Bioenergetics/mitochondrial metabolism | Phosphorylates glucose to G6P, couples glycolysis to OXPHOS on the OMM by interacting with VDAC and ANT |
| KIF1B | CMT2A1 (118210) | Autosomal dominant | Mitochondrial trafficking | Monomeric motor protein that performs anterograde axonal mitochondrial transport |
| KIF5A | SPG10 (604187) | Autosomal dominant | Mitochondrial trafficking | Kinesin-1 motor protein that performs anterograde axonal transport of mitochondria |
| MFN2 | CMT2A (609260) CMT2A (617087) CMT5 CMT6 (601152) |
AD AR AD AD |
Mitochondrial fusion/MAMs interaction | OMM-localized fusion protein, forms a tethering complex by homologous interaction with ER-localized MFN2 |
| MT-ATP6 | CMT2 | Autosomal dominant | Bioenergetics | ATP6 subunit of ATP synthase (OXPHOS complex V) |
| NEFL | CMT1F (607734) CMT2E (607684) |
Autosomal dominant | Mitochondrial shape/ mitochondrial trafficking | Neuron-specific component of the cytoskeleton that regulates mitochondrial shape and motility |
| OPA1 | Optic atrophy (165500) Optic atrophy plus (125250) |
Autosomal dominant | Mitochondrial fusion/bioenergetics | A mitochondrial fusion protein that also remodels cristae |
| OPA3 | Optic atrophy (165300) Costeff syndrome (258501) |
AD AR |
Mitochondrial fission/bioenergetics | Localizes to the OMM and regulates lipid metabolism, thermogenesis, and mitochondrial fission |
| PDK3 | CMTX6 (300905) | X-linked | Bioenergetics | Phosphorylates PDC, inactivating its activity, shutting down pyruvate-derived acetyl-CoA entry into the TCA |
| SIGMAR1 | dHMN | Autosomal recessive | MAMs interaction | ER chaperone protein that maintains calcium exchange between ER and mitochondria |
| SLC25A46 | CMT2 Leigh syndrome Optic atrophy |
Autosomal recessive | Mitochondrial fusion/MAMs interaction/bioenergetics | Transmembrane protein localized to OMM, mediates lipid transfer between ER and mitochondria, maintains mitochondrial lipid homeostasis and cristae |
| TUBB3 | CFEOM3 (600638) | Autosomal dominant | Mitochondrial trafficking | Polymerizes to form axonal microtubules |
| VAPB | ALS8 (608627) SMA (182980) |
Autosomal dominant | MAMs interaction | Regulates autophagosome formation at MAMs through VAPB-PTPIP51 complex |
Abbreviations: AD = autosomal dominant; ALS8 = amyotrophic lateral sclerosis 8; ANT = adenine nucleotide translocator; AIFM1 = apoptosis inducing factor mitochondria associated 1; AR = autosomal recessive; CFEOM3 = extraocular muscles type 3; CMT = Charcot-Marie-Tooth disease; dHMN = distal hereditary motor neuropathy; DHTKD1 = dehydrogenase E1 and transketolase domain containing 1; DNM1L = dynamin 1 like; DYNC1H1 = dynein cytoplasmic 1 heavy chain 1; FIS1 = fission 1 protein; G6P = glucose-6-phosphate; GDAP1 = ganglioside induced differentiation associated protein 1; HK1 = hexokinase 1; IMS = intermembrane space; KIF1B = kinesin-3 isoform B; KIF5A = kinesin-1 isoform A; MFN2 = mitofusin 2; MT-ATP6 = mitochondrially encoded ATP synthase 6; NEFL = neurofilament, light polypeptide; OMM = outer mitochondrial membrane; OPA1 = optic atrophy 1; OPA3 = optic atrophy 3; OXPHOS = oxidative phosphorylation; PDC = pyruvate dehydrogenase complex; PDK3 = pyruvate dehydrogenase kinase 3; PTPIP51 = protein tyrosine phosphatase-interacting protein 51; SIGMAR1 = sigma non-opioid intracellular receptor 1; SLC25A46 = solute carrier family 25 member 46; SMA = spinal muscular atrophy; SMALED = spinal muscular atrophy, lower extremity predominant; SPG10 = spastic paraplegia 10; TCA = tricarboxylic acid cycle; TUBB3 = class III β-tubulin; VAPB = vesicle associated membrane protein-associated protein B; VDAC = voltage-dependent anion channel.
3.1. CMT, Hereditary Motor Neuropathies and Hereditary Sensory Neuropathies
CMT is a heterogeneous group of inherited neuropathies that affect both sensory and motor neurons and is also known as hereditary motor and sensory neuropathy (HMSN). Classically CMT is divided into a demyelinating form of the disease (CMT1, based on slowed motor nerve conduction velocities) and an axonal form (CMT2, based on normal nerve conduction velocities). In both CMT1 and CMT2 and their multiple respective subtypes, both motor and sensory nerves are affected. Clinical manifestations vary and include distal muscle wasting and weakness with sensory loss that most commonly begins distally in the lower limbs and progresses with age proximally, to include the upper limbs. Over 70 mutations are identified as underlying distinct forms of CMT neuropathies as well as two forms of CMT2, distal hereditary motor neuropathies (dHMN), and hereditary sensory neuropathies (HSN), which predominantly affect motor and sensory nerves, respectively (Pareyson et al., 2015; Rossor, Polke, Houlden, & Reilly, 2013). The nomenclature of the distinct types of CMT neuropathies continues to evolve even today. Historically these neuropathies were classified on a purely clinical presentation and nerve conduction studies; currently CMT neuropathies are classified based on gene mutation(s) coupled with clinical phenotypes (Pisciotta & Shy, 2018; Rossor, Tomaselli, & Reilly, 2016). Regardless, the unifying thread across all inherited mutations is that they adversely impact cellular machinery that impairs neurons and axons (Rossor et al., 2013). These impairments include defects in mitochondrial function, ER homeostasis, axonal transport, and synaptic transmission. Mutations to mitochondrial proteins or mitochondrial motor proteins lead to altered mitochondrial bioenergetics, fission/fusion, and trafficking that make PNS neurons and axons especially susceptible to injury and degeneration (Table 1).
3.1.1. CMT mutations in genes that regulate mitochondrial bioenergetics
Altered mitochondrial bioenergetics reduce ATP output (Section 2.2), and since neurons are reliant on a constant ATP source (Section 2.1), this scenario has negative consequences for neuronal health and survival. Mutations to several metabolic mitochondrial enzymes underlie inherited peripheral neuropathies. Apoptosis inducing factor mitochondria associated 1 (AIFM1) is an NADH oxidase encoded in the nucleus that is transported to the mitochondria (Rinaldi et al., 2012). Mutant AIFM1 is associated with recessive X-linked CMTX4 otherwise known as Cowchock syndrome [Online Mendelian Inheritance in Man (OMIM)# 310490], which manifests with axonal neuropathy, deafness, and cognitive impairment (Rinaldi et al., 2012). Other peripheral neuropathy disorders have also been linked with mutant AIFM1, a rare severe, early-onset mitochondrial encephalopathy combined with OXPHOS deficiency (COXPD6, OMIM# 300816) (Ghezzi et al., 2010) and a rare infantile motor neuron disease (Diodato et al., 2016).
Mutation to another metabolic mitochondrial protein, dehydrogenase E1 and transketolase domain containing 1 (DHTKD1), is associated with CMT2Q (OMIM# 615025), which is similar to classical CMT2 (W. Y. Xu et al., 2012). DHTKD1 encodes the E1 subunit of the mitochondrial amino acid degrading alpha-ketoadipic acid dehydrogenase complex (Hagen et al., 2015). Knockdown of DHTKD1 in vitro decreased ATP production and mitochondrial biogenesis while increasing ROS generation, leading to a greater extent of cellular apoptosis and lower proliferation (W. Xu et al., 2013). Hexokinase 1 (HK1) phosphorylates glucose to glucose-6-phosphate (G6P), the initiation in most glucose metabolic pathways. A fraction of HK1 is bound to the OMM, where it couples cytoplasmic glycolysis to OXPHOS by interacting with VDAC and the IMM-bound adenine nucleotide translocator (ANT) (Robey & Hay, 2006). Mutant HK1 is linked with CMT4G (OMIM# 605285) and also a severe early-onset CMT1, which is autosomal recessively inherited (Gabrikova et al., 2013).
Mitochondrial encoded ATP synthase 6 (MT-ATP6) is a mitochondrial DNA gene coding for the ATP6 subunit of mitochondrial ATP synthase of complex V in OXPHOS. Mutations in MT-ATP6 leads to a form of CMT2 (Pitceathly et al., 2012) or other neuropathies, such as NARP syndrome (neurogenic muscle weakness, ataxia, and retinitis pigmentosa) (Thorburn, Rahman, & Rahman, 1993). The NARP MT-ATP6 mutation results in lower ATP production despite proper complex assembly and normal protein expression levels (Rak et al., 2007). Another metabolic mitochondrial enzyme, pyruvate dehydrogenase kinase 3 (PDK3), is involved in peripheral neuropathy. PDK3 phosphorylates PDC, the multi-enzyme complex that generates acetyl-CoA from pyruvate coupling glycolysis to the TCA cycle (Gudi, Bowker-Kinley, Kedishvili, Zhao, & Popov, 1995). Phosphorylation of PDC thoroughly inactivates its enzymatic activity, shutting down pyruvate-derived acetyl-CoA entry into the TCA cycle. Mutant PDK3 is associated with dominant X-linked inheritance CMTX6 (OMIM# 300905) (Kennerson et al., 2016; Kennerson et al., 2013).
3.1.2. CMT mutations in genes that regulate mitochondrial morphology, fission, and fusion
Mitochondria reshape, divide, and fuse in response to a cell’s energy needs (Section 2.3), and interference with this carefully regulated process is inevitably detrimental to cellular health. Mutation to mitochondrial fission and fusion proteins are well established causes of CMT. The most prevalent mutation in CMT patients in a mitochondrial protein is MFN2. Mutations in MFN2 results in CMT2A (OMIM# 609260) (Pareyson et al., 2015). Mutant MFN2 is also associated with autosomal recessive CMT2A (OMIM# 617087) and with autosomal dominant CMT5, a rare category characterized by pyramidal tract involvement (D. Zhu et al., 2005), and CMT6 (HSMN6, OMIM# 601152), which is accompanied by optic atrophy (Zuchner et al., 2006). Other complex phenotypes also arise more akin to mitochondrial encephalopathies (Boaretto et al., 2010). The majority of MFN2-linked CMT patients display a severe phenotype with early onset sensory and motor neuropathy and rapid progression although a fraction exhibit less debilitating disease. Aside from neuropathy, patients harboring mutant MFN2 may also suffer from tremors, proximal weakness, respiratory involvement, optic atrophy, hearing loss, vocal cord palsy, and spotty white matter brain abnormalities (Pareyson et al., 2015).
Around 100 mutant MFN2 variants have been identified, mostly as missense mutations in essential protein domains (e.g., GTPase domain, coil-coiled motifs), yet the precise mechanism that leads from mutant MFN2 to CMT2 pathology and neuropathy is not precisely known, nor why it manifests itself in a cell-specific manner. Certain mutant MFN2 variants negatively impact mitochondrial fusion (A. L. Misko, Sasaki, Tuck, Milbrandt, & Baloh, 2012), yet others have no effect and complementation with wild-type MFN1 can rescue fusion defects (Detmer & Chan, 2007). Axonal transport may similarly be compromised by mutant MFN2 (A. L. Misko et al., 2012), possibly through an interaction with Miro (A. Misko, Jiang, Wegorzewska, Milbrandt, & Baloh, 2010), as well as through loss of calcium homeostasis or ROS production.
Mutant GDAP1 is another major contributor to CMT (Cassereau, Chevrollier, Gueguen, et al., 2011), possibly the second most common type of autosomal recessive CMT and perhaps approximately 5% of all CMT2 cases (Pareyson et al., 2015). Mutations to GDAP1 give rise to CMT subsets, either as an autosomal recessive, demyelinating CMT4A (OMIM# 214400), which is characterized by early onset, possibly with vocal cord and diaphragmatic paralysis (OMIM# 607706) (Baxter et al., 2002; Claramunt et al., 2005), or an autosomal recessive, axonal, early onset, severe CMT2K (OMIM# 607831) or autosomal dominant, late onset, less severe CMT2K (OMIM# 607831) (Cassereau, Chevrollier, Bonneau, et al., 2011).
Examination of sural nerve biopsies from patients diagnosed with dominant CMT2K confirm mitochondrial defects, characterized by aggregates of normal and abnormal mitochondria (Sivera et al., 2010). Similar mitochondrial abnormalities are a common feature in nerve biopsies from both dominant and recessive CMT2K patients (Fu et al., 2017). Dominant and recessive GDAP1 mutations may adversely impact mitochondrial dynamics (Niemann et al., 2009). Overexpression of recessive mutant GDAP1 in HeLa cells reduced mitochondrial fission whereas dominant mutant GDAP1 inhibited fusion and increased ROS production and susceptibility to apoptosis. In CMT2K patient-derived fibroblasts, dominant mutant GDAP1 induced a 40% decrease in complex I turnover and a 33 and 20% decrease in tubular mitochondria size and mitochondrial mass, respectively, suggesting dominant mutant GDAP1 may impact mitochondrial bioenergetics as well as morphology (Cassereau et al., 2009). In addition to altered bioenergetics, another facet of research related to mutant GDAP1 in CMT4A and CMT2K is dysregulation of calcium dynamics (Gonzalez-Sanchez et al., 2017; Gonzalez-Sanchez, Satrustegui, Palau, & Del Arco, 2019). GDAP1 possesses putative glutathione S-transferase (GST) binding sites and a possible role against oxidative stress has also been proposed for wild-type GDAP1 (Noack et al., 2012).
3.1.3. CMT mutations in genes that regulate MAMs
MAMs are crucial to healthy mitochondrial function (Section 2.4), and mutations to several proteins essential for maintaining MAMs are associated with CMT (Bernard-Marissal et al., 2018). MFN2 forges MAM tethering complexes in addition to its role in mitochondrial fusion. Although most reports have focused on its impact on mitochondrial fusion in CMT2A, a recent study examined MAM dynamics in fibroblasts derived from 3 CMT2 patients harboring mutant MFN2 (Larrea et al., 2019). Mitochondria-ER contact distance and ER-mitochondria contact coefficient (a measure of ER-mitochondrial distance, length of contact, and mitochondrial perimeter), MAM-specific phospholipid synthesis, and capacitative calcium entry (extracellular calcium influx upon intracellular depletion) were impacted to varying extents by mutant MFN2 depending on the disease phenotype severity. On the other hand, respiratory chain function was relatively unaffected, leading the authors to conclude that mutant MFN2 predominantly affects MAMs but not mitochondrial bioenergetics.
SIGMAR1 is an ER chaperone protein that maintains calcium exchange between ER and mitochondria (Hayashi et al., 2009), and mutations to this gene have been linked to autosomal recessively inherited dHMN (Gregianin et al., 2016; Li et al., 2015). In vitro functional studies in human neuroblastoma cell lines transfected with mutant SIGMAR1 exhibited defects in SIGMAR1 localization, which did not accumulate at MAMs, but instead formed aggregates (Gregianin et al., 2016). Mutant SIGMAR1 also decreased basal proliferation and survival under stress and altered cellular calcium dynamics. Autophagy was induced to a greater extent in mutant SIGMAR1 cells, but the autophagosomes were not mobilized for clearing the SIMGAR1 aggregates. Another study similarly found that expression of mutant SIGMAR1 led to ER stress and elevated apoptosis (Li et al., 2015).
Solute carrier family 25 member 46 (SLC25A46) is a transmembrane protein located in the OMM that mediates lipid transfer between ER and mitochondria (Palmieri, 2013). It maintains mitochondrial lipid homeostasis and cristae; loss of function SLC25A46 mutations inhibit mitochondrial respiration and trigger mitochondrial hyperfusion (Janer et al., 2016). Mutations to SLC25A46 are linked to CMT2 and optic atrophy (Abrams et al., 2015) and to Leigh syndrome (Janer et al., 2016). Regulation of autophagy is another important MAM function; autophagosomes occur at MAMs in mammalian cells (Hamasaki et al., 2013). Autophagy related 14 (ATG14) and ATG5 localize to MAMs under starvation conditions, a process that is blocked in cells with disrupted MAMs through MFN2 knockdown. The tethering complex VAPB-PTPIP51 also regulates autophagy at MAMs (Gomez-Suaga et al., 2017); VAPB or PTPIP51 overexpression tighten MAMs, whereas knockdown loosens them, initiating autophagosome formation. The impact of VAPB-PTPIP51 on autophagy is specifically dependent on their tethering function and involves their role in calcium transport from ER to mitochondria. The role of autophagy, including autophagy at MAMs, is increasingly appreciated in hereditary peripheral neuropathies (Haidar & Timmerman, 2017). Although no mutation to either VAPB or PTPIP51 has been linked to CMT, mutations to VAPB have been identified in patients with motor neuron diseases, including ALS (type 8, OMIM# 608627) and spinal muscular atrophy (OMIM# 182980) (Nishimura et al., 2004).
3.1.4. CMT mutations in genes that regulate mitochondrial trafficking
Interference with the mitochondrial transport machinery (Section 2.5) jeopardizes a neuron’s capacity to deliver mitochondria to areas of high energy demand. The occurrence of heritable neuropathies with mutations to key mitochondrial transport proteins highlights the essential nature of mitochondrial trafficking to neuronal health. Mutations to the key anterograde mitochondrial transport motor KIF5A causes hereditary spastic paraplegia 10 (SPG10, OMIM# 604187), an autosomal dominant disorder characterized by progressive gait disorder and spasticity accompanied by degeneration of corticospinal tracts and peripheral nerves (Reid et al., 2002). The disease phenotype can vary and can present as a forme-fruste of axonal CMT2 (Crimella et al., 2012). In a similar vein, mutation to kinesin-3 isoform B (KIF1B), a monomeric motor for anterograde mitochondrial transport (Nangaku et al., 1994), has been identified in a single family with CMT2A1 (OMIM# 118210) (C. Zhao et al., 2001). Another isoform, KIF1A, is a neuron-specific kinesin, which transports vesicles rather than mitochondria. Mutant KIF1A also leads to rare, recessively inherited neuropathies, HSN2C (OMIM# 614213) (Riviere et al., 2011) or SPG30 (OMIM# 610357) (Erlich et al., 2011).
Mutations to the retrograde dynein motor and its accessory proteins also cause heritable neuropathies. Dynein cytoplasmic 1 heavy chain 1 (DYNC1H1) is a dynein subunit; mutant DYNC1H1 is linked with dominant CMT2O (OMIM# 614228) (Weedon et al., 2011) as well as dominant SMALED (spinal muscular atrophy, lower extremity predominant, OMIM# 158600), a disorder of cortical migration defects, intellectual disability, and lower-limb predominant abnormalities (Harms et al., 2012). In mice, heterozygous mutant DYNC1H1 results in progressive motor neuron degeneration (Hafezparast et al., 2003) and deficient retrograde transport (J. Zhao et al., 2016). Fibroblasts derived from heterozygous mutant DYNC1H1 mice display aberrant mitochondrial morphology, similar to SMALED patient-derived fibroblasts, and progressive mitochondrial dysfunction (Eschbach et al., 2013).
In addition to mutations to the motor proteins, mutations to the tracts along which mitochondria are transported can similarly lead to neuropathy. Class III β-tubulin (β3-tubulin or β-tubulin III), is a microtubule isotype of the tubulin family, which is mostly specific to neurons and is encoded by the TUBB3 gene (Sullivan & Cleveland, 1986). Mutant TUBB3 results in congenital fibrosis of the extraocular muscles type 3 (CFEOM3, OMIM# 600638), an axonal sensorimotor polyneuropathy with loss of eye movement (Hong et al., 2015; Tischfield et al., 2010). In vivo in a knock-in mouse model, mutant TUBB3 triggers deficiencies in axon guidance but not in cortical cell migration (Tischfield et al., 2010). In vitro, it interferes with tubulin heterodimerization, but mutant heterodimers that fold retain the ability to polymerize. In yeast, certain mutant TUBB3 variants disrupt microtubule dynamics while others perturb the microtubule-kinesin interaction. Dynamin 2 (DNM2) is a dynamin-like GTPase also linked to CMT; however, the involvement of this microtubule associated protein to mitochondrial dynamics has not been fully determined (Pareyson et al., 2015).
Mutation to cytoskeletal proteins have also been linked with mitochondrial disorders in inherited neuropathies. Mutant NEFL occurs in a generally autosomal dominant CMT1F (OMIM# 607734) or CMT2E (OMIM# 607684), with patients frequently suffering from early onset and severe disease, and whose sural nerve biopsies show accumulated mitochondria (Fabrizi et al., 2007). Indeed, NFs impact mitochondrial dynamics; mutant NEFL results in attenuated mitochondrial fusion and increased movement in primary mouse dorsal root ganglion (DRG) neurons (Gentil et al., 2012). Also, in mouse DRG neurons, expression of mutant NEFL resulted in rounder and shorter mitochondria preceding disruption of the neurofilament network, suggesting mitochondrial dysfunction contributes to CMT2E pathogenesis (Tradewell, Durham, Mushynski, & Gentil, 2009). Aberrant mitochondrial trafficking was also seen in motor neurons differentiated from induced pluripotent stem cells obtained from CMT2E patient fibroblasts (Saporta et al., 2015).
3.2. Other hereditary neuropathies
Mutant OPA1 is linked to autosomal dominant optic atrophy (OMIM# 165500), which is primarily characterized by optic nerve deterioration leading to progressive vision loss (Alexander et al., 2000; Delettre et al., 2000). Around 20% of patients additionally exhibit peripheral neuropathy and are classified as autosomal dominant optic atrophy plus (OMIM# 125250) (Burte, Carelli, Chinnery, & Yu-Wai-Man, 2015). Optic atrophy 3 (OPA3) is a protein that localizes to the OMM, that regulates lipid metabolism and thermogenesis (Wells et al., 2012), and triggers mitochondrial fission (Ryu, Jeong, Choi, Karbowski, & Choi, 2010). Autosomal dominant mutant OPA3 leads to optic atrophy and premature cataracts (OMIM# 165300) (Reynier et al., 2004), whereas autosomal recessive mutant variants are associated with 3-methylglutaconic aciduria type III (alternatively called Costeff syndrome, OMIM# 258501) (Anikster, Kleta, Shaag, Gahl, & Elpeleg, 2001). OPA3 overexpression in cells markedly increases mitochondrial fragmentation while knockdown results in mitochondrial elongation, demonstrating an impact of OPA3 on mitochondrial dynamics (Ryu et al., 2010). Mutant DNM1L results in severe infantile encephalopathy (OMIM# 614388) (Waterham et al., 2007) by altering DNM1L recruitment and oligomerization and hence mitochondrial fission (Chang et al., 2010; Fahrner, Liu, Perry, Klein, & Chan, 2016). Familial ALS patients harbor mutations to mitochondrial trafficking proteins that trigger neuropathy, including coiled-coil-helix-coiled-coil-helix domain containing 10 gene (CHCHD10), SIGMAR1, VDAC1, and VAPB (Bannwarth et al., 2014; Bernard-Marissal et al., 2018). A broad spectrum of additional rare neuropathies with mitochondrial defects have been documented but are outside the scope of this review. The reader is directed to other excellent reviews for further reading (Cassereau, Codron, & Funalot, 2014; Pareyson, Piscosquito, Moroni, Salsano, & Zeviani, 2013).
3.3. Neuropathy associated with diabetes and prediabetes
Hereditary neuropathies that exhibit mitochondrial dysfunction have provided important considerations for evaluating mitochondrial dynamics in neuropathy associated with diabetes and prediabetes. The genetic mutations to OXPHOS complexes, fission and fusion proteins, MAM proteins, and mitochondrial trafficking motor proteins in hereditary neuropathies suggest that similar pathways may also be altered in neuropathy associated with diabetes and prediabetes. Of critical importance to these studies, however, is the recent data from a Cochrane review of clinical trials with diabetic patients exhibiting neuropathy as an outcome measure. The results showed that maintaining tight glycemic control improved neuropathy outcomes in type 1 diabetes (T1D) patients but had limited efficacy in slowing the progression of neuropathy in T2D patients (Callaghan, Little, Feldman, & Hughes, 2012). Instead, a follow-up study reported that neuropathy development in T2D patients correlates with components of metabolic syndrome, including obesity and dyslipidemia (Callaghan & Feldman, 2014; Callaghan, Xia, Banerjee, et al., 2016; Lupachyk, Watcho, Hasanova, Julius, & Obrosova, 2012). Prediabetic patients with metabolic syndrome also develop neuropathy and the progression of nerve damage and sensory loss is identical to their T2D counterparts (Callaghan, Xia, Reynolds, et al., 2016). Therefore, there may be differential disease mechanisms related to hyperglycemia and dyslipidemia underlying the development of neuropathy in T1D and T2D patients, respectively (Callaghan, Hur, & Feldman, 2012). Hence, while dysfunctional mitochondrial pathways are only beginning to be addressed in the context of diabetes and prediabetes, Section 4 will highlight the effect of hyperglycemia on mitochondrial mechanisms underlying neuropathy development, and Section 5 will focus on the impact of dyslipidemia on mitochondrial function and dynamics associated with the development of neuropathy.
4. Regulation of mitochondrial dynamics by glucose
4.1. Hyperglycemia compromises DRG neuron bioenergetics
Elevated levels of glucose associated with hyperglycemia are processed by glycolysis and the TCA cycle followed by OXPHOS (Feldman et al., 2017). Excess glucose levels overload the glycolytic and TCA cycle resulting in a high proton gradient across the IMM. OXPHOS becomes compromised in the high proton gradient resulting in a decrease in ATP generation and increase in ROS production by an overloaded electron transport system.
Hyperglycemia significantly impairs mitochondrial bioenergetics in cultured DRG neurons. Elevated levels of glucose associated with hyperglycemia leads to excessive flux through the glycolytic pathway. Treatment of DRG neurons with 100 mM glucose decreases mitochondrial respiration and reduces ATP turnover (Rumora et al., 2018). Lower ATP production resulting from less efficient mitochondrial respiration suggests that DRG neuron bioenergetics are compromised by hyperglycemia. In combination with these altered bioenergetics, an increase in mitochondrial DNA indicates that mitochondrial biogenesis is occurring in DRG neurons, most likely to compensate for the increased metabolic load.
In parallel, DRG neurons from TD1 mice and rats show compromised maximal oxygen consumption rate and diminished spare respiratory capacity (S. K. Chowdhury et al., 2010; S. R. Chowdhury et al., 2014). This decrease in mitochondrial respiratory function is accompanied by a decrease in mitochondrial electron transport chain proteins complexes I and IV and the TCA cycle protein citrate synthase (S. K. Chowdhury et al., 2010). Interestingly, mitochondrial respiration can be recovered using insulin (Aghanoori et al., 2017; S. K. Chowdhury et al., 2010), neurotrophin-3 (Huang, Sayers, Verkhratsky, & Fernyhough, 2005), or ciliary neurotrophic factor (S. R. Chowdhury et al., 2014), which correlates with improved hind paw thermal sensitivity.
4.2. Hyperglycemia induces aberrant mitochondrial fission in DRG axons
As discussed above, DRG neurons respond to oxidative stress through mitochondrial biogenesis allowing DRG neurons to increase mitochondrial mass by the division of existing mitochondria (Vincent et al., 2010). Increased mitochondrial mass is thought to improve metabolic capacity and minimize mitochondrial damage. However, excessive levels of glucose lead to proapoptotic fission of the mitochondria. Proapoptotic fission is facilitated by DNM1L which splits the mitochondria and recruits proapoptotic protein BCL2 associated X (BAX) (Leinninger, Backus, et al., 2006). This recruitment is thought to prevent mitochondrial fusion and permeabilize the mitochondrial membrane for apoptosis (Leinninger, Edwards, Lipshaw, & Feldman, 2006). Knockdown of fission protein DNM1L in glucose-treated DRG neurons results in a decrease in mitochondrial fission and an improvement in neuronal health. Collectively, these data suggest that apoptotic fission may play a key role in glucose-induced injury in DRG neurons (Russell et al., 2002; Vincent, McLean, Backus, & Feldman, 2005).
4.3. Potential for hyperglycemia to regulate ER-mitochondrial interactions DRG neurons
Although glucose-regulated MAMs have not been reported in DRG neurons or diabetic nerve damage, MAM interactions are essential for intracellular metabolic pathways and are likely to play a key role in the progression of neuropathy, as documented for the hereditary neuropathies (Section 3.1.3). Numerous reports link hyperglycemia to MAM formation in the regulation of mitochondrial function. Elevated concentrations of glucose trigger the formation of fragmented mitochondria and a decrease in MAM interactions in liver, skeletal muscle, adipose tissue, and beta cells (Rieusset et al., 2016; Theurey et al., 2016). Further research is needed in this area to understand the role of MAMs in diabetic and prediabetic neuropathy.
4.4. Hyperglycemia has no effect on mitochondrial trafficking in DRG axons
Despite the reduction in mitochondrial respiration and ATP production in glucose-treated DRG neurons, glucose has no effect on mitochondrial motility in the axons of DRG neurons cultured from adult mice (Rumora et al., 2018). Additionally, the directionality and velocity of mitochondrial transport is not altered by glucose treatment. These results suggest that axonal mitochondrial transport is maintained despite impaired mitochondrial bioenergetics. Interestingly, mitochondrial transport is altered in DRG neurons treated with dyslipidemic concentrations of LCSFAs (Section 5.5) indicating that mitochondrial transport is differentially affected by hyperglycemia and dyslipidemia.
In contrast to DRG neurons, glucose metabolism in hippocampal neurons of the central nervous system (CNS) plays a crucial role in regulating axonal mitochondrial transport through post-translational modification of the motor protein Milton. Rat hippocampal neurons treated with physiological levels of glucose display GlcNacylation of Milton by O-GlcNAc transferase resulting in a significant reduction in axonal mitochondrial transport (Pekkurnaz, Trinidad, Wang, Kong, & Schwarz, 2014). This glucose-related post-translational regulation of axonal mitochondrial transport is specific to mitochondria in hippocampal neurons. These results suggest that DRG neurons may possess unique regulatory mechanisms for mitochondrial dynamics and mitochondrial transport that differ from neurons of the central nervous system. Moreover, the effect of hyperglycemia and dyslipidemia on molecular mechanisms are distinct in DRG neurons.
5. Regulation of mitochondrial dynamics by dyslipidemia
5.1. Dyslipidemia and neuropathy
Since dyslipidemia plays a central role in the pathogenesis of neuropathy in patients with prediabetes and T2D (Callaghan, Little, et al., 2012), current studies are focused on understanding the lipid-related disease mechanisms that contribute to neuropathy development. To identify pathogenic molecular mechanisms underlying the development of neuropathy, murine models of prediabetes and T2D have been used to model dysfunctional mitochondrial dynamics. Common models of T2D include leptin and leptin receptor knockout animals, the ob/ob and db/db murine models respectively, characterized by hyperglycemia, dyslipidemia, and polyneuropathy. Prediabetes is also modeled in mice, using a lard-based high fat diet (HFD) chow. HFD-fed mice develop prediabetes characterized by obesity, dyslipidemia, and neuropathy similar to their human counterparts (O’Brien, Sakowski, & Feldman, 2014).
The fatty acid composition of murine diets has recently emerged as a regulator of nerve function in prediabetic and diabetic murine models. Recent studies by the Yorek laboratory demonstrate that polyunsaturated fatty acids (PUFAs) reduce diabetes- and prediabetes-induced neuropathy. Mice fed a HFD combined with streptozotocin treatment develop T2D and neuropathy. Feeding these mice menhaden oil, an oil composed of omega-3 PUFAs, alone (Shevalye et al, 2015) or in combination with α-lipoic acid, an antioxidant, and enalapril, an angiotensin-converting enzyme inhibitor (Yorek et al., 2017), improves neuropathy phenotypes compared to untreated animals. In a rat model of T2D, menhaden oil similarly slowed nerve loss and neuropathy progression (Coppey, Davidson, Shevalye, Torres, & Yorek, 2018). Cumulatively, the results suggest that LCSFAs are pathogenic in the development of neuropathy whereas PUFAs can prevent neuropathy in prediabetic or diabetic mice.
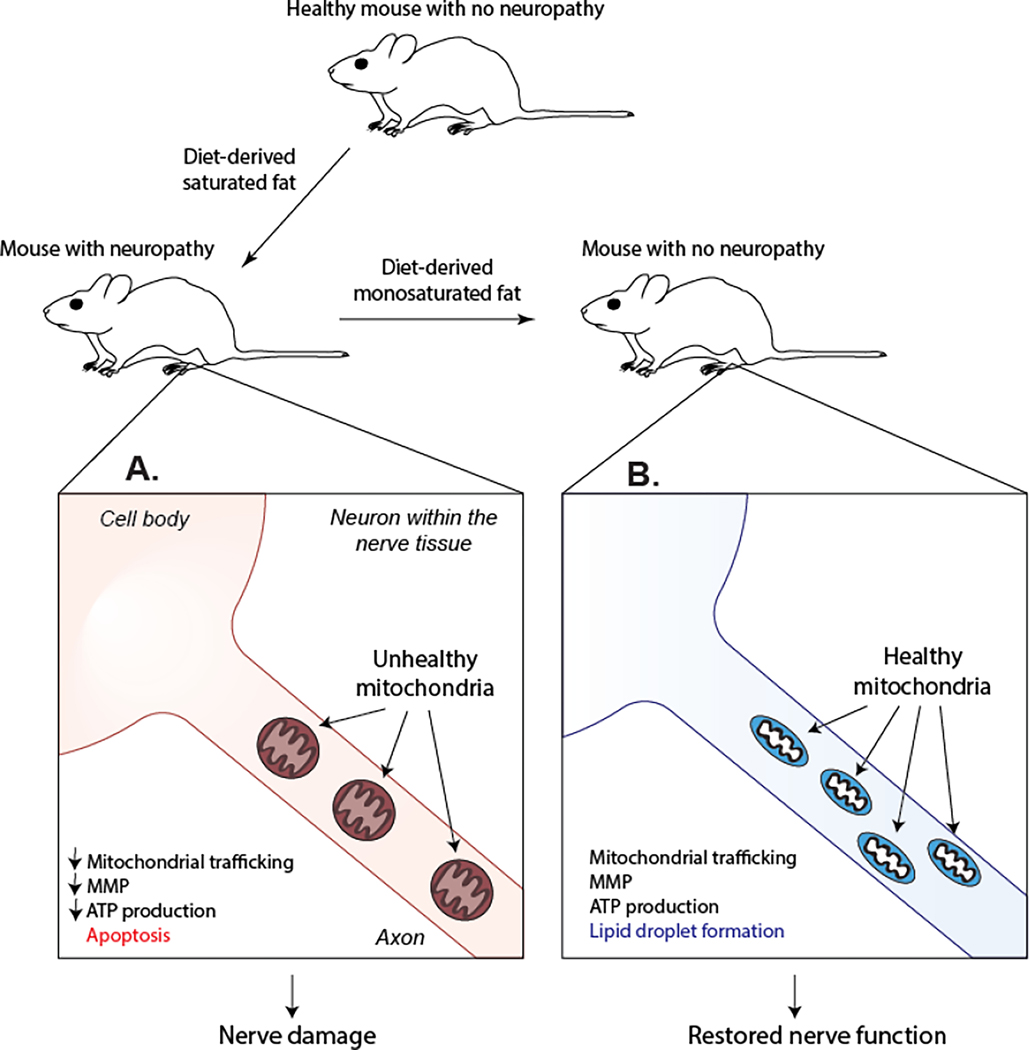
In line with these studies, monounsaturated fatty acids (MUFAs) also restore nerve function in a murine model of prediabetes (Rumora, LoGrasso, Hayes, et al., 2019) (Fig. 2). Mice fed a LCSFA-rich HFD develop glucose intolerance, increased body weight and fat mass and exhibit deficits in both sensory and motor nerve conduction velocity. A separate group of mice fed the LCSFA-rich HFD followed by 8 weeks of a MUFA-rich HFD (HFD-MUFA) exhibited similar metabolic dysfunction but had a complete restoration in sensory and motor nerve conduction velocities. HFD-MUFA-fed mice also exhibited a significant increase in intraepidermal nerve fiber density in footpads compared to animals who remained on the LCSFA-rich HFD. These results suggest that unsaturated fatty acids have a beneficial effect on nerve function and are likely to improve mitochondrial dynamics to repair peripheral nerve function.
Fig. 2.
A dietary switch from a SFA-rich HFD to a MUFA-rich HFD restores nerve function in a murine model of prediabetes and neuropathy. A) Mice fed a SFA-rich HFD develop robust neuropathy phenotypes including a decrease in sensory and motor nerve conduction velocity and a reduction in intraepidermal nerve fiber density. In line with these results, cultured DRG neurons treated with SFAs exhibit a decrease in axonal mitochondrial trafficking and MMP. These mitochondrial changes are associated with apoptosis and a reduction in neuronal ATP level. B) Changing mice from a SFA-rich diet to a MUFA-rich diet restores both nerve and mitochondrial function.
5.2. Dyslipidemia and mitochondrial bioenergetics
Our laboratory has recently reported that treating db/db T2D mice with pioglitazone, an agonist of the peroxisome proliferator-activated receptor gamma (PPAR-γ), improves diabetic neuropathy. Transcriptomic analyses of sciatic nerves from treated animals reveal changes in expression patterns of genes related to OXPHOS, FA β-oxidation, and the TCA cycle, suggesting that improving bioenergetic pathways correlates with an improvement in nerve function (Hinder et al., 2017). Additionally, impairment of mitochondrial bioenergetics in the sciatic nerves of db/db mice and DRG neurons of HFD-fed mice indicate that mitochondrial energy production is compromised under dyslipidemic conditions (Sas et al., 2016; Sas et al., 2018).
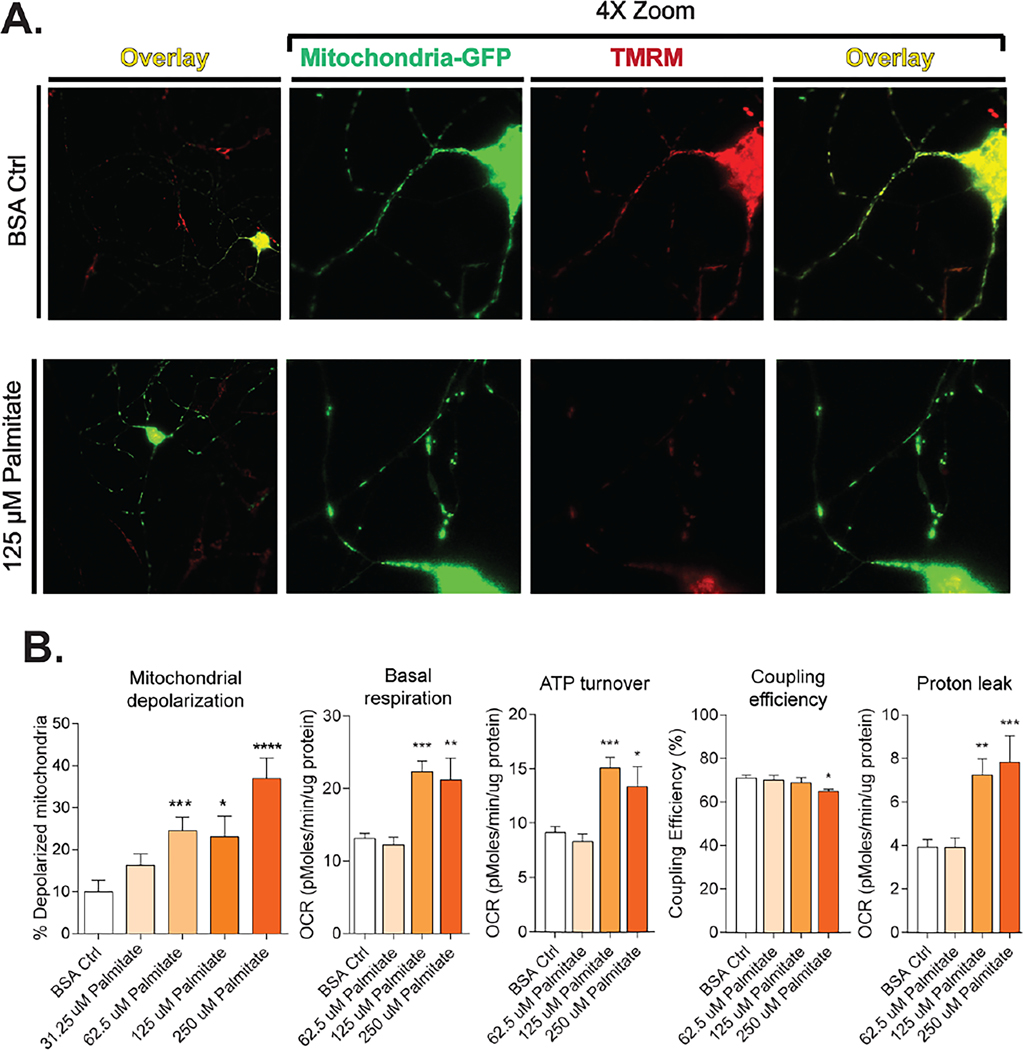
To support these findings, an in vitro analysis of mitochondrial bioenergetics in DRG neurons revealed that diabetic levels of LCSFA palmitate caused an elevation in resting ATP generation and basal respiration (Rumora et al., 2018) (Fig. 3B). These changes in resting state bioenergetics were accompanied by a significant proton leak. Diabetic levels of LCSFA palmitate caused significant impairment of OXPHOS. These changes correlated with palmitate-induced axonal mitochondrial depolarization (Rumora et al., 2018) and of intracellular loss of ATP level (Rumora, LoGrasso, Haidar, et al., 2019).
Fig. 3.
Dyslipidemia decreases mitochondrial function in DRG neurons. (A) LCSFA palmitate induces a loss of MMP through mitochondrial depolarization. Mitochondria labeled with mito-GFP are stained with MMP-dependent stain TMRM. Axonal mitochondria maintain MMP in the bovine serum albumin vehicle control and appear yellow in an overlay of both signals. Palmitate-treated cells exhibit a loss in TMRM staining due to mitochondrial depolarization. (B) Palmitate impairs mitochondrial bioenergetics through mitochondrial depolarization by increasing basal respiration and ATP turn over which leads to a decrease in coupling efficiency and increased proton leak from the electron transport chain. Bioenergetics bar graphs included with permission from Rumora, A.E., Lentz, S.I., Hinder, L.M., Jackson, S.W., Valesano, A., Levinson, G.E., and Feldman, E.L. (2018). Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. Faseb j 32, 195–207. Mitochondrial depolarization figures adapted with permission from Rumora, A.E., LoGrasso, G., Haidar, J.A., Dolkowski, J.J., Lentz, S.I., and Feldman, E.L. (2019). Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res 60, 58–70.
Lower bioenergetic energy production reduces axonal ATP levels in the nerve and affects essential ATP-driven cellular mechanisms, such as axonal mitochondrial transport (Rumora et al., 2018). The loss of functional mitochondria in the axon is likely to exacerbate the reduction in axonal ATP leading to complete bioenergetic failure (De Vos & Hafezparast, 2017). Therefore, research efforts are being directed toward understanding how certain diet-derived fatty acids are responsible for dysfunction in DRG neuron bioenergetics and these fatty acids effect neuronal and axonal mitochondrial trafficking and mitochondrial function.
5.3. Fusion and fission dynamics in dyslipidemia
Since mitochondrial bioenergetics are dependent on maintaining a balance of mitochondrial dynamics, fission and fusion of mitochondria are likely to play a key role in the pathogenesis of neuropathy. Although few studies have focused on fission and fusion in the dyslipidemic nerve, lipids are emerging as regulators of mitochondrial morphology and size. Complex lipids that are upregulated in dyslipidemia such as cardiolipin, phosphatidic acid, diacylglycerol, and phosphatidylethanolamine can regulate mitochondrial morphology (Frohman, 2015; Ha & Frohman, 2014). Cardiolipin is a negatively charged lipid found within the IMM that interacts with fusion and fission proteins. It is required for mitochondrial fusion mediated by dynamin-related GTPase MGM1 (yeast; mitochondrial dynamin like GTPase OPA1 in humans), a protein localized on the IMM (DeVay et al., 2009). Cardiolipin may also interact with α-synuclein to induce mitochondrial fission (Nakamura et al., 2011), and also with pro-apoptotic factors, such as BAX and BH3 interacting domain death agonist (BID), which it recruits to mitochondria (Lucken-Ardjomande, Montessuit, & Martinou, 2008; Lutter et al., 2000).
Phosphatidylethanolamine is another phospholipid present within the OMM and IMM that controls membrane curvature and mitochondrial fusion. Yeast cells that lack phosphatidylserine decarboxylase 1, the enzyme that synthesizes phosphatidylethanolamine, exhibit fragmented mitochondria (Chan & McQuibban, 2012). Phosphatidic acid, which can be derived from cardiolipin, is also a negatively charged lipid that induces curvature and induces mitochondrial aggregation and fusion through DNM1L (Adachi et al., 2016; Ha & Frohman, 2014). Conversely, diacylglycerol induces mitochondrial fission, but the precise mechanism is not known (Ha & Frohman, 2014). Dyslipidemia-induced alterations to phospholipid synthesis could potentially alter mitochondrial size and morphology by changing the mitochondrial membrane lipid composition. Indeed, in vitro in mouse DRG neurons, palmitate and stearate increased mitochondrial circularity and shifted their size distribution towards larger mitochondria (Rumora, LoGrasso, Haidar, et al., 2019).
5.4. ER-mitochondrial interactions in dyslipidemia
Although the role of MAMs in prediabetic and diabetic neuropathy is unknown (Section 4.3), MAMs are upregulated in response to metabolic overload associated with prediabetes and T2D (Arruda et al., 2014; Lowell & Shulman, 2005). MAMs have been identified in murine models of obesity and are essential for regulating lipid synthesis pathways and maintaining mitochondrial function. MAMs are a major site for lipid biosynthesis and lipid exchange between the ER and mitochondria. The two major lipid synthesized at the MAM site are phospholipids, phosphatidylcholine and phosphatidylethanolamine (Rowland & Voeltz, 2012). Additionally, MAM formation can play a role in ceramide transfer from the ER to the mitochondria to initiate apoptotic cell death. MAMs are also major regulatory sites for regulating calcium-driven cell death. Under cellular homeostasis, calcium transfer from the ER to the mitochondria preserves normal bioenergetics (Cardenas et al., 2010). An increase of calcium flux from the ER to the mitochondria at the MAM site can trigger apoptosis (Section 2.4). Therefore, future studies on the involvement of MAMs in dyslipidemia and neuropathy may provide a novel therapeutic target for neuropathy.
5.5. The effect of dyslipidemia on axonal mitochondrial trafficking
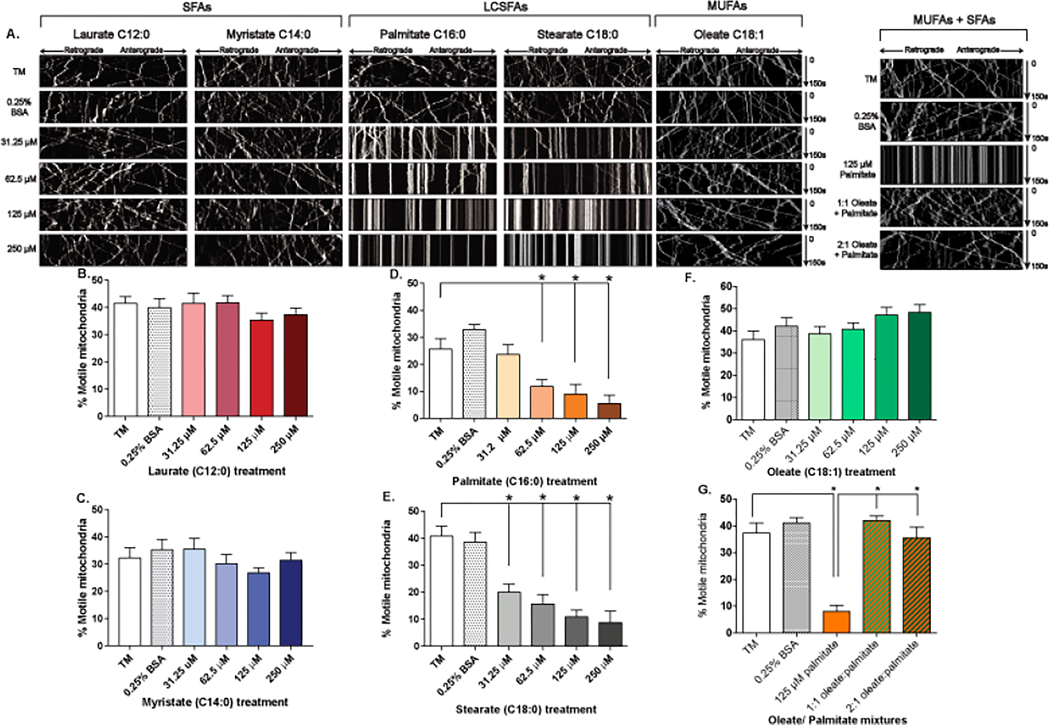
A number of recent experiments have specifically investigated the differential impact of LCSFAs and MUFAs on mitochondrial function and trafficking in sensory neurons. Treatment of primary mouse DRG neurons with the LCSFA palmitate (C16:0) (Fig. 4D) substantially lowered the number and velocity of motile mitochondria and increased mitochondrial depolarization (Fig. 3A,B) (Rumora et al., 2018). Similar outcomes were reported upon incubation of primary mouse DRG neurons with the LCSFA stearate (C18:0) (Fig. 4E), which markedly reduced the fraction of motile mitochondria and velocity of mitochondrial trafficking (Rumora, LoGrasso, Haidar, et al., 2019). Conversely, the shorter LCSFA myristate (C14:0) (Fig. 4C) and the medium chain saturated fatty acid (MCSFA) laurate (C12:0) (Fig. 4B) did not elicit any changes to mitochondrial trafficking. The LCSFA-induced impairment in mitochondrial trafficking correlated with a reduction in mitochondrial membrane potential, decrease in ATP levels, and activation of neuronal apoptosis (Rumora, LoGrasso, Haidar, et al., 2019) (Fig 3A,B). Overall, this body of evidence suggests LCSFAs trigger mitochondrial dysfunction supporting a role for dyslipidemia in the pathogenesis of neuropathy.
Fig. 4.
SFAs impair axonal mitochondrial transport in DRG neurons. (A) A kymograph analysis is used to record mitochondrial movement from 0–150 seconds using live-cell confocal microscopy. Straight lines indicate non-motile mitochondria. Elevated levels of exogenous short and medium chain SFAs laurate (B) and myristate (C) did not impact the number of motile mitochondrial in the axon of sensory DRG neurons. LCSFAs associated with T2D and prediabetes such as palmitate (D) and stearate (E) induce a dose-dependent decrease in the percentage of motile mitochondria in the axon of sensory DRG neurons. (F) MUFA oleate had no effect on mitochondrial motility. (G) Mixtures of oleate and palmitate prevented palmitate-induced impairment of mitochondrial transport. SFA kymographs and bar graphs modified with permission from Rumora, A.E., LoGrasso, G., Haidar, J.A., Dolkowski, J.J., Lentz, S.I., and Feldman, E.L. (2019). Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res 60, 58–70. Permission to be obtained for MUFA kymographs and bar graphs from J. Neurosci upon release of paper in press.
Another study compared the effect of MUFA oleate and LCSFA palmitate on mitochondrial trafficking to determine the beneficial effect of dietary MUFAs in prediabetic murine models of neuropathy (Rumora, LoGrasso, Hayes, et al., 2019). As previously reported, palmitate caused a significant reduction in the percentage of motile mitochondria in the axons of DRG neurons. Conversely, oleate had no effect on mitochondrial trafficking (Fig. 4F). Interestingly, equimolar or 2:1 mixture of oleate and palmitate completely prevented the palmitate-induced impairment in mitochondrial transport (Fig. 4G). Oleate also prevented mitochondrial depolarization, reduction in ATP levels, and caspase activation. The mechanism by which oleate prevents mitochondrial dysfunction may be related to lipid droplet formation throughout the DRG axon (Fig. 2B). The formation of lipid droplets allows for the sequestration of palmitate preventing downstream mitochondrial dysfunction.
This differential regulation of axonal mitochondrial trafficking in DRG axons suggests that LCSFAs including palmitate and stearate are particularly damaging to DRG neurons. It is likely that LCSFAs are metabolized into lipotoxic lipids, such as ceramides (Listenberger et al., 2003), diacylglycerols, or cardiolipins, that cause mitochondrial dysfunction and apoptosis in DRG neurons and axons. A MUFA-rich HFD can reverse neuropathy and restore nerve function. On a molecular level, oleate prevents impairment of mitochondrial axonal transport, retains mitochondrial membrane potential, and abolishes DRG neuron apoptosis likely by the formation of intra-axonal lipid droplets that sequester lipotoxic LCSFAs (Fig. 2B).
6. Conclusions
Peripheral neuronal architecture is essential for transmitting sensory and motor signals across long distances between the CNS and PNS, but it places unique constraints on managing their energy demands. First, neurons rely on mitochondria during basal and high activity periods for their constant ATP demand (i.e., mitochondrial bioenergetics). Second, in order to meet that continuous need, neurons are reliant on carefully orchestrated mitochondrial dynamics to ensure balanced biogenesis (i.e., fission/fusion, remodeling) for optimal energy production, and that these mitochondria are delivered to areas of high activity (i.e., trafficking/transport). Interactions with other organelles such as ER (i.e., MAMs) add a further layer of coordinated activity, in addition to clearance mechanism, which are necessary for removing and replacing damaged mitochondria. Heritable peripheral neuropathies caused by mutations in genes that regulate mitochondrial bioenergetics, fission/fusion, and transport elegantly demonstrate this critical vulnerability of neurons. Loss of well-regulated mitochondrial dynamics in these heritable neuropathies leads to aberrant mitochondrial functioning and neuronal injury and loss, underscoring the essential role of mitochondria in normal nerve function.
This particular susceptibility of neurons to mitochondrial dysfunction has led to investigations into their roles in the pathogenesis of neuropathy associated with prediabetes and diabetes. Confirming this, studies have shown that mitochondrial dysfunction underlies neuropathy onset and development in both prediabetic and diabetic patients. Hyperglycemia and dyslipidemia contribute to the pathogenesis of neuropathy but exert a differential effect on neuronal mitochondrial dynamics and function. Preliminary results suggest that impaired mitochondrial bioenergetics in prediabetes or diabetes plays a key role in the progression of neuropathy by reducing axonal energy to maintain neuronal function. To compensate for this bioenergetic reduction, DRG neurons undergo mitochondrial biogenesis under conditions of hyperglycemia or proton leak during dyslipidemia. Elevated LCSFAs associated with dyslipidemia impair mitochondrial trafficking, depolarize the MPP, induce a loss of ATP, and apoptosis. Conversely, MUFAs restore mitochondrial dynamics in DRG axons and nerve function in murine models of neuropathy. These recent developments in mitochondrial mechanisms underlying the progression of neuropathy will direct future studies with the goal of developing treatments for this prevalent and debilitating complication.
Acknowledgments
The original work presented in this chapter was supported by the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R24 DK082841 and R01 DK107956 (to E.L.F.) and F32 1F32DK112642 and T32 1T32DK101357 (to A.E.R.); the NIDDK DiaComp Award DK076169 (to E.L.F); Novo Nordisk Foundation Grant NNF14OC0011633 (to E.L.F.); the Milstein, Nathan and Rose Research Fund; the American Diabetes Association; the Program for Neurology Research and Discovery; and the A. Alfred Taubman Medical Research Institute.
References
- Abrams AJ, Hufnagel RB, Rebelo A, Zanna C, Patel N, Gonzalez MA, . . . Dallman JE (2015). Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat Genet, 47(8), 926–932. doi: 10.1038/ng.3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Itoh K, Yamada T, Cerveny KL, Suzuki TL, Macdonald P, . . . Sesaki H. (2016). Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division. Mol Cell, 63(6), 1034–1043. doi: 10.1016/j.molcel.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghanoori MR, Smith DR, Roy Chowdhury S, Sabbir MG, Calcutt NA, & Fernyhough P. (2017). Insulin prevents aberrant mitochondrial phenotype in sensory neurons of type 1 diabetic rats. Exp Neurol, 297, 148–157. doi: 10.1016/j.expneurol.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, . . . Wissinger B. (2000). OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet, 26(2), 211–215. doi: 10.1038/79944 [DOI] [PubMed] [Google Scholar]
- Angelova PR, & Abramov AY (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett, 592(5), 692–702. doi: 10.1002/1873-3468.12964 [DOI] [PubMed] [Google Scholar]
- Anikster Y, Kleta R, Shaag A, Gahl WA, & Elpeleg O. (2001). Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am J Hum Genet, 69(6), 1218–1224. doi: 10.1086/324651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, & Hotamisligil GS (2014). Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med, 20(12), 1427–1435. doi: 10.1038/nm.3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, & Milbrandt J. (2007). Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci, 27(2), 422–430. doi: 10.1523/jneurosci.4798-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannwarth S, Ait-El-Mkadem S, Chaussenot A, Genin EC, Lacas-Gervais S, Fragaki K, . . . Paquis-Flucklinger V. (2014). A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain, 137(Pt 8), 2329–2345. doi: 10.1093/brain/awu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RV, Ben Othmane K, Rochelle JM, Stajich JE, Hulette C, Dew-Knight S, . . . Vance JM (2002). Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet, 30(1), 21–22. doi: 10.1038/ng796 [DOI] [PubMed] [Google Scholar]
- Bernard-Marissal N, Chrast R, & Schneider BL (2018). Endoplasmic reticulum and mitochondria in diseases of motor and sensory neurons: a broken relationship? Cell Death Dis, 9(3), 333. doi: 10.1038/s41419-017-0125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet AM, Delerue T, Millet AM, Moulis MF, David C, Daloyau M, . . . Belenguer P. (2016). Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis, 90, 3–19. doi: 10.1016/j.nbd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Boaretto F, Vettori A, Casarin A, Vazza G, Muglia M, Rossetto MG, . . . Martinuzzi A. (2010). Severe CMT type 2 with fatal encephalopathy associated with a novel MFN2 splicing mutation. Neurology, 74(23), 1919–1921. doi: 10.1212/WNL.0b013e3181e240f9 [DOI] [PubMed] [Google Scholar]
- Boyer PD (1997). The ATP synthase--a splendid molecular machine. Annu Rev Biochem, 66, 717–749. doi: 10.1146/annurev.biochem.66.1.717 [DOI] [PubMed] [Google Scholar]
- Brickley K, & Stephenson FA (2011). Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J Biol Chem, 286(20), 18079–18092. doi: 10.1074/jbc.M111.236018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burte F, Carelli V, Chinnery PF, & Yu-Wai-Man P. (2015). Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol, 11(1), 11–24. doi: 10.1038/nrneurol.2014.228 [DOI] [PubMed] [Google Scholar]
- Callaghan BC, & Feldman EL (2014). Painful diabetic neuropathy: many similarly effective therapies with widely dissimilar costs. Ann Intern Med, 161(9), 674–675. doi: 10.7326/M14-2157 [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Hur J, & Feldman EL (2012). Diabetic neuropathy: one disease or two? Curr Opin Neurol, 25(5), 536–541. doi: 10.1097/WCO.0b013e328357a797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Little AA, Feldman EL, & Hughes RA (2012). Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev(6), CD007543. doi: 10.1002/14651858.CD007543.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Price RS, & Feldman EL (2015). Distal Symmetric Polyneuropathy: A Review. Jama, 314(20), 2172–2181. doi: 10.1001/jama.2015.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Xia R, Banerjee M, de Rekeneire N, Harris TB, Newman AB, . . . Health ABCS(2016). Metabolic Syndrome Components Are Associated With Symptomatic Polyneuropathy Independent of Glycemic Status. Diabetes Care, 39(5), 801–807. doi: 10.2337/dc16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, . . . Feldman EL (2016). Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol, 73(12), 1468–1476. doi: 10.1001/jamaneurol.2016.3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, . . . Foskett JK (2010). Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell, 142(2), 270–283. doi: 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Bonneau D, Verny C, Procaccio V, Reynier P, & Ferre M. (2011). A locus-specific database for mutations in GDAP1 allows analysis of genotype-phenotype correlations in Charcot-Marie-Tooth diseases type 4A and 2K. Orphanet J Rare Dis, 6, 87. doi: 10.1186/1750-1172-6-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Gueguen N, Desquiret V, Verny C, Nicolas G, . . . Procaccio V. (2011). Mitochondrial dysfunction and pathophysiology of Charcot-Marie-Tooth disease involving GDAP1 mutations. Exp Neurol, 227(1), 31–41. doi: 10.1016/j.expneurol.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Gueguen N, Malinge MC, Letournel F, Nicolas G, . . . Reynier P. (2009). Mitochondrial complex I deficiency in GDAP1-related autosomal dominant Charcot-Marie-Tooth disease (CMT2K). Neurogenetics, 10(2), 145–150. doi: 10.1007/s10048-008-0166-9 [DOI] [PubMed] [Google Scholar]
- Cassereau J, Codron P, & Funalot B. (2014). Inherited peripheral neuropathies due to mitochondrial disorders. Rev Neurol (Paris), 170(5), 366–374. doi: 10.1016/j.neurol.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Cecchini G. (2003). Function and structure of complex II of the respiratory chain. Annu Rev Biochem, 72, 77–109. doi: 10.1146/annurev.biochem.72.121801.161700 [DOI] [PubMed] [Google Scholar]
- Chaban Y, Boekema EJ, & Dudkina NV (2014). Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta, 1837(4), 418–426. doi: 10.1016/j.bbabio.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Chan EY, & McQuibban GA (2012). Phosphatidylserine decarboxylase 1 (Psd1) promotes mitochondrial fusion by regulating the biophysical properties of the mitochondrial membrane and alternative topogenesis of mitochondrial genome maintenance protein 1 (Mgm1). J Biol Chem, 287(48), 40131–40139. doi: 10.1074/jbc.M112.399428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, & Blackstone C. (2010). Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci, 1201, 34–39. doi: 10.1111/j.1749-6632.2010.05629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, Manlandro CM, Arnoult D, Stadler J, Posey AE, Hill RB, & Blackstone C. (2010). A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. J Biol Chem, 285(42), 32494–32503. doi: 10.1074/jbc.M110.142430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, & Sheng ZH (2013). Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol, 202(2), 351–364. doi: 10.1083/jcb.201302040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrollier A, Guillet V, Loiseau D, Gueguen N, de Crescenzo MA, Verny C, . . . Reynier P. (2008). Hereditary optic neuropathies share a common mitochondrial coupling defect. Ann Neurol, 63(6), 794–798. doi: 10.1002/ana.21385 [DOI] [PubMed] [Google Scholar]
- Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, . . . Fernyhough P. (2010). Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes, 59(4), 1082–1091. doi: 10.2337/db09-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SR, Saleh A, Akude E, Smith DR, Morrow D, Tessler L, . . . Fernyhough P. (2014). Ciliary neurotrophic factor reverses aberrant mitochondrial bioenergetics through the JAK/STAT pathway in cultured sensory neurons derived from streptozotocin-induced diabetic rodents. Cell Mol Neurobiol, 34(5), 643–649. doi: 10.1007/s10571-014-0054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claramunt R, Pedrola L, Sevilla T, Lopez de Munain A, Berciano J, Cuesta A, . . . Palau F. (2005). Genetics of Charcot-Marie-Tooth disease type 4A: mutations, inheritance, phenotypic variability, and founder effect J Med Genet (Vol. 42, pp. 358–365). England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, & Caceres A. (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci, 10(5), 319–332. doi: 10.1038/nrn2631 [DOI] [PubMed] [Google Scholar]
- Coppey L, Davidson E, Shevalye H, Torres ME, & Yorek MA (2018). Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab Syndr Obes, 11, 117–127. doi: 10.2147/dmso.s159071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez M, Singleton JR, & Smith AG (2014). Glucose intolerance, metabolic syndrome, and neuropathy. Handb Clin Neurol, 126, 109–122. doi: 10.1016/B978-0-444-53480-4.00009-6 [DOI] [PubMed] [Google Scholar]
- Crimella C, Baschirotto C, Arnoldi A, Tonelli A, Tenderini E, Airoldi G, . . . Bassi MT (2012). Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot-Marie-Tooth type 2. Clin Genet, 82(2), 157–164. doi: 10.1111/j.1399-0004.2011.01717.x [DOI] [PubMed] [Google Scholar]
- Crofts AR (2004). The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol, 66, 689–733. doi: 10.1146/annurev.physiol.66.032102.150251 [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, . . . Hajnoczky G. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol, 174(7), 915–921. doi: 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza K, Nzirorera C, & Kienesberger PC (2016). Lipid metabolism and signaling in cardiac lipotoxicity. Biochim Biophys Acta, 1861(10), 1513–1524. doi: 10.1016/j.bbalip.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, . . . Votruba M. (2007). Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet, 16(11), 1307–1318. doi: 10.1093/hmg/ddm079 [DOI] [PubMed] [Google Scholar]
- de Brito OM, & Scorrano L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature, 456(7222), 605–610. doi: 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, . . . Grierson AJ(2007). Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet, 16(22), 2720–2728. doi: 10.1093/hmg/ddm226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, & Hafezparast M. (2017). Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol Dis, 105, 283–299. doi: 10.1016/j.nbd.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Morotz GM, Stoica R, Tudor EL, Lau KF, Ackerley S, . . . Miller CC (2012). VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet, 21(6), 1299–1311. doi: 10.1093/hmg/ddr559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA (2010). Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia, 53(7), 1270–1287. doi: 10.1007/s00125-010-1684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, . . . Hamel CP (2000). Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet, 26(2), 207–210. [DOI] [PubMed] [Google Scholar]
- Detmer SA, & Chan DC (2007). Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol, 176(4), 405–414. doi: 10.1083/jcb.200611080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, & Nunnari J. (2009). Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol, 186(6), 793–803. doi: 10.1083/jcb.200906098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diodato D, Tasca G, Verrigni D, D’Amico A, Rizza T, Tozzi G, . . . Bertini E. (2016). A novel AIFM1 mutation expands the phenotype to an infantile motor neuron disease. Eur J Hum Genet, 24(3), 463–466. doi: 10.1038/ejhg.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Y, Edvardson S, Hodges E, Zenvirt S, Thekkat P, Shaag A, . . . Elpeleg O. (2011). Exome sequencing and disease-network analysis of a single family implicate a mutation in KIF1A in hereditary spastic paraparesis. Genome Res, 21(5), 658–664. doi: 10.1101/gr.117143.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach J, Sinniger J, Bouitbir J, Fergani A, Schlagowski AI, Zoll J, . . . Dupuis L(2013). Dynein mutations associated with hereditary motor neuropathies impair mitochondrial morphology and function with age. Neurobiol Dis, 58, 220–230. doi: 10.1016/j.nbd.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, . . . Rizzuto N. (2007). Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain, 130(Pt 2), 394–403. doi: 10.1093/brain/awl284 [DOI] [PubMed] [Google Scholar]
- Fahrner JA, Liu R, Perry MS, Klein J, & Chan DC (2016). A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy. Am J Med Genet A, 170(8), 2002–2011. doi: 10.1002/ajmg.a.37721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Nave KA, Jensen TS, & Bennett DLH (2017). New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron, 93(6), 1296–1313. doi: 10.1016/j.neuron.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, & Aspenstrom P. (2006). The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun, 344(2), 500–510. doi: 10.1016/j.bbrc.2006.03.163 [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, & Voeltz GK (2011). ER tubules mark sites of mitochondrial division. Science, 334(6054), 358–362. doi: 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA (2015). Role of mitochondrial lipids in guiding fission and fusion. J Mol Med (Berl), 93(3), 263–269. doi: 10.1007/s00109-014-1237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Dai S, Lu Y, Wu R, Wang Z, Yuan Y, & Lv H. (2017). Similar clinical, pathological, and genetic features in Chinese patients with autosomal recessive and dominant Charcot-Marie-Tooth disease type 2K. Neuromuscul Disord, 27(8), 760–765. doi: 10.1016/j.nmd.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Fucho R, Casals N, Serra D, & Herrero L. (2017). Ceramides and mitochondrial fatty acid oxidation in obesity. Faseb j, 31(4), 1263–1272. doi: 10.1096/fj.201601156R [DOI] [PubMed] [Google Scholar]
- Gabrikova D, Mistrik M, Bernasovska J, Bozikova A, Behulova R, Tothova I, & Macekova S. (2013). Founder mutations in NDRG1 and HK1 genes are common causes of inherited neuropathies among Roma/Gypsies in Slovakia. J Appl Genet, 54(4), 455–460. doi: 10.1007/s13353-013-0168-7 [DOI] [PubMed] [Google Scholar]
- Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, . . . Dillmann W. (2012). Modulation of Dynamin-related Protein 1 (DRP1) Function by Increased O-linked-β-N-acetylglucosamine Modification (O-GlcNAc) in Cardiac Myocytes. Journal of Biological Chemistry, 287(35), 30024–30034. doi: 10.1074/jbc.M112.390682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmetti V, De Rosa P, Torosantucci L, Marini ES, Romagnoli A, Di Rienzo M, . . . Valente EM (2017). PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy, 13(4), 654–669. doi: 10.1080/15548627.2016.1277309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil BJ, Minotti S, Beange M, Baloh RH, Julien JP, & Durham HD (2012). Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-Tooth disease. Faseb j, 26(3), 1194–1203. doi: 10.1096/fj.11-196345 [DOI] [PubMed] [Google Scholar]
- Gentil BJ, Tibshirani M, & Durham HD (2015). Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res, 360(3), 609–620. doi: 10.1007/s00441-014-2082-7 [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Sevrioukova I, Invernizzi F, Lamperti C, Mora M, D’Adamo P, . . . Zeviani M. (2010). Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am J Hum Genet, 86(4), 639–649. doi: 10.1016/j.ajhg.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, & Miller CCJ (2017). The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr Biol, 27(3), 371–385. doi: 10.1016/j.cub.2016.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez P, Pla-Martin D, Martinez-Valero P, Rueda CB, Calpena E, Del Arco A, . . . Satrustegui J. (2017). CMT-linked loss-of-function mutations in GDAP1 impair store-operated Ca(2+) entry-stimulated respiration. Sci Rep, 7, 42993. doi: 10.1038/srep42993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez P, Satrustegui J, Palau F, & Del Arco A. (2019). Calcium Deregulation and Mitochondrial Bioenergetics in GDAP1-Related CMT Disease. Int J Mol Sci, 20(2). doi: 10.3390/ijms20020403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregianin E, Pallafacchina G, Zanin S, Crippa V, Rusmini P, Poletti A, . . . Vazza G. (2016). Loss-of-function mutations in the SIGMAR1 gene cause distal hereditary motor neuropathy by impairing ER-mitochondria tethering and Ca2+ signalling. Hum Mol Genet, 25(17), 3741–3753. doi: 10.1093/hmg/ddw220 [DOI] [PubMed] [Google Scholar]
- Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, & Popov KM (1995). Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem, 270(48), 28989–28994. [DOI] [PubMed] [Google Scholar]
- Ha EEJ, & Frohman MA (2014). Regulation of mitochondrial morphology by lipids. BioFactors (Oxford, England), 40(4), 419–424. doi: 10.1002/biof.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, . . . Fisher EM (2003). Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science, 300(5620), 808–812. doi: 10.1126/science.1083129 [DOI] [PubMed] [Google Scholar]
- Hagen J, te Brinke H, Wanders RJ, Knegt AC, Oussoren E, Hoogeboom AJ, . . . Houten SM (2015). Genetic basis of alpha-aminoadipic and alpha-ketoadipic aciduria. J Inherit Metab Dis, 38(5), 873–879. doi: 10.1007/s10545-015-9841-9 [DOI] [PubMed] [Google Scholar]
- Haidar M, & Timmerman V. (2017). Autophagy as an Emerging Common Pathomechanism in Inherited Peripheral Neuropathies. Front Mol Neurosci, 10, 143. doi: 10.3389/fnmol.2017.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, . . . Yoshimori T. (2013). Autophagosomes form at ER-mitochondria contact sites. Nature, 495(7441), 389–393. doi: 10.1038/nature11910 [DOI] [PubMed] [Google Scholar]
- Harms MB, Ori-McKenney KM, Scoto M, Tuck EP, Bell S, Ma D, . . . Baloh RH (2012). Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology, 78(22), 1714–1720. doi: 10.1212/WNL.0b013e3182556c05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, & Attwell D. (2012). Synaptic energy use and supply. Neuron, 75(5), 762–777. doi: 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, & Su TP (2009). MAM: more than just a housekeeper. Trends Cell Biol, 19(2), 81–88. doi: 10.1016/j.tcb.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder LM, Park M, Rumora AE, Hur J, Eichinger F, Pennathur S, . . . Feldman EL (2017). Comparative RNA-Seq transcriptome analyses reveal distinct metabolic pathways in diabetic nerve and kidney disease. J Cell Mol Med, 21(9), 2140–2152. doi: 10.1111/jcmm.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, & Brady ST (1991). Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol, 114(2), 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, & Takemura R. (2005). Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci, 6(3), 201–214. doi: 10.1038/nrn1624 [DOI] [PubMed] [Google Scholar]
- Hirst J. (2005). Energy transduction by respiratory complex I--an evaluation of current knowledge. Biochem Soc Trans, 33(Pt 3), 525–529. doi: 10.1042/bst0330525 [DOI] [PubMed] [Google Scholar]
- Hong YB, Lee JH, Park HJ, Choi YR, Hyun YS, Park JH, . . . Choi BO (2015). A family with axonal sensorimotor polyneuropathy with TUBB3 mutation. Mol Med Rep, 11(4), 2729–2734. doi: 10.3892/mmr.2014.3047 [DOI] [PubMed] [Google Scholar]
- Houten SM, & Wanders RJ (2010). A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis, 33(5), 469–477. doi: 10.1007/s10545-010-9061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TJ, Sayers NM, Verkhratsky A, & Fernyhough P. (2005). Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol, 194(1), 279–283. doi: 10.1016/j.expneurol.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, & Grimm S. (2011). Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. Embo j, 30(3), 556–568. doi: 10.1038/emboj.2010.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer A, Prudent J, Paupe V, Fahiminiya S, Majewski J, Sgarioto N, . . . Shoubridge EA (2016). SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol Med, 8(9), 1019–1038. doi: 10.15252/emmm.201506159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia R, Wang RY, Yusuf A, Thomas PV, Agard DA, Shaw JM, & Frost A. (2018). Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature, 558(7710), 401–405. doi: 10.1038/s41586-018-0211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, & Sheng ZH (2008). Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell, 132(1), 137–148. doi: 10.1016/j.cell.2007.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastaniotis AJ, Autio KJ, Keratar JM, Monteuuis G, Makela AM, Nair RR, . . . Hiltunen JK. (2017). Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim Biophys Acta Mol Cell Biol Lipids, 1862(1), 39–48. doi: 10.1016/j.bbalip.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Kawaguchi K. (2013). Role of kinesin-1 in the pathogenesis of SPG10, a rare form of hereditary spastic paraplegia. Neuroscientist, 19(4), 336–344. doi: 10.1177/1073858412451655 [DOI] [PubMed] [Google Scholar]
- Kennerson ML, Kim EJ, Siddell A, Kidambi A, Kim SM, Hong YB, . . . Choi BO (2016). X-linked Charcot-Marie-Tooth disease type 6 (CMTX6) patients with a p.R158H mutation in the pyruvate dehydrogenase kinase isoenzyme 3 gene. J Peripher Nerv Syst, 21(1), 45–51. doi: 10.1111/jns.12160 [DOI] [PubMed] [Google Scholar]
- Kennerson ML, Yiu EM, Chuang DT, Kidambi A, Tso SC, Ly C, . . . Nicholson GA (2013). A new locus for X-linked dominant Charcot-Marie-Tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene. Hum Mol Genet, 22(7), 1404–1416. doi: 10.1093/hmg/dds557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, & Higgs HN (2013). An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science, 339(6118), 464–467. doi: 10.1126/science.1228360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HA, & Johnson WA (1937). Metabolism of ketonic acids in animal tissues. Biochem J, 31(4), 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrea D, Pera M, Gonelli A, Cabrera RQ, Akman HO, Guardia-Laguarta C, . . . Giacomello M. (2019). MFN2 mutations in Charcot-Marie-Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum Mol Genet. doi: 10.1093/hmg/ddz008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, & Lu B. (2014). The myriad roles of Miro in the nervous system: axonal transport of mitochondria and beyond. Front Cell Neurosci, 8, 330. doi: 10.3389/fncel.2014.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, & Feldman EL (2006). Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiology of Disease, 23, 11–22. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Edwards JL, Lipshaw MJ, & Feldman EL (2006). Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol, 2(11), 620–628. [DOI] [PubMed] [Google Scholar]
- Li X, Hu Z, Liu L, Xie Y, Zhan Y, Zi X, . . . Zhang R. (2015). A SIGMAR1 splice-site mutation causes distal hereditary motor neuropathy. Neurology, 84(24), 2430–2437. doi: 10.1212/wnl.0000000000001680 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr., Ory DS, & Schaffer JE (2003). Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A, 100(6), 3077–3082. doi: 10.1073/pnas.0630588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau D, Chevrollier A, Verny C, Guillet V, Gueguen N, Pou de Crescenzo MA, . . . Reynier P. (2007). Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Ann Neurol, 61(4), 315–323. doi: 10.1002/ana.21086 [DOI] [PubMed] [Google Scholar]
- Lopez-Crisosto C, Bravo-Sagua R, Rodriguez-Pena M, Mera C, Castro PF, Quest AF, . . . Lavandero S. (2015). ER-to-mitochondria miscommunication and metabolic diseases. Biochim Biophys Acta, 1852(10 Pt A), 2096–2105. doi: 10.1016/j.bbadis.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, & Chan DC (2013). Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell, 24(5), 659–667. doi: 10.1091/mbc.E12-10-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, & Shulman GI (2005). Mitochondrial dysfunction and type 2 diabetes. Science, 307(5708), 384–387. doi: 10.1126/science.1104343 [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, & Martinou JC (2008). Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ, 15(5), 929–937. doi: 10.1038/cdd.2008.9 [DOI] [PubMed] [Google Scholar]
- Lupachyk S, Watcho P, Hasanova N, Julius U, & Obrosova IG (2012). Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative-nitrosative stress. Free Radic Biol Med, 52(8), 1255–1263. doi: 10.1016/j.freeradbiomed.2012.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie X, & Wang X. (2000). Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol, 2(10), 754–761. doi: 10.1038/35036395 [DOI] [PubMed] [Google Scholar]
- Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, & Dillmann WH (2011). Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol, 300(6), R1296–1302. doi: 10.1152/ajpregu.00437.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, & Pinton P. (2014). The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta, 1837(4), 461–469. doi: 10.1016/j.bbabio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, & Nunnari J. (2006). Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell, 127(2), 383–395. doi: 10.1016/j.cell.2006.09.021 [DOI] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, & Nunnari J. (2004). Mitochondrial fusion intermediates revealed in vitro. Science, 305(5691), 1747–1752. doi: 10.1126/science.1100612 [DOI] [PubMed] [Google Scholar]
- Miller KE, & Sheetz MP (2004). Axonal mitochondrial transport and potential are correlated. J Cell Sci, 117(Pt 13), 2791–2804. doi: 10.1242/jcs.01130 [DOI] [PubMed] [Google Scholar]
- Mishra P, & Chan DC (2016). Metabolic regulation of mitochondrial dynamics. J Cell Biol, 212(4), 379–387. doi: 10.1083/jcb.201511036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, & Baloh RH (2010). Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci, 30(12), 4232–4240. doi: 10.1523/jneurosci.6248-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko AL, Sasaki Y, Tuck E, Milbrandt J, & Baloh RH (2012). Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci, 32(12), 4145–4155. doi: 10.1523/jneurosci.6338-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, . . . Zorzano A. (2013). Mfn2 modulates the UPR and mitochondrial function via repression of PERK. Embo j, 32(17), 2348–2361. doi: 10.1038/emboj.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, . . . Edwards RH (2011). Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem, 286(23), 20710–20726. doi: 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, & Hirokawa N. (1994). KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell, 79(7), 1209–1220. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, . . . Shaw JM (2014). Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A, 111(35), E3631–3640. doi: 10.1073/pnas.1402449111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, & Budd SL (2000). Mitochondria and neuronal survival. Physiol Rev, 80(1), 315–360. doi: 10.1152/physrev.2000.80.1.315 [DOI] [PubMed] [Google Scholar]
- Niemann A, Wagner KM, Ruegg M, & Suter U. (2009). GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis, 36(3), 509–520. doi: 10.1016/j.nbd.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Nirschl JJ, Ghiretti AE, & Holzbaur ELF (2016). Lipid Rafts Assemble Dynein Ensembles. Trends Biochem Sci, 41(5), 393–394. doi: 10.1016/j.tibs.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, . . . Zatz M. (2004). A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet, 75(5), 822–831. doi: 10.1086/425287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack R, Frede S, Albrecht P, Henke N, Pfeiffer A, Knoll K, . . . Methner A. (2012). Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum Mol Genet, 21(1), 150–162. doi: 10.1093/hmg/ddr450 [DOI] [PubMed] [Google Scholar]
- O’Brien PD, Hinder LM, Callaghan BC, & Feldman EL (2017). Neurological consequences of obesity. Lancet Neurol, 16(6), 465–477. doi: 10.1016/s1474-4422(17)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, Hinder LM, Sakowski SA, & Feldman EL (2014). ER stress in diabetic peripheral neuropathy: A new therapeutic target. Antioxid Redox Signal, 21(4), 621–633. doi: 10.1089/ars.2013.5807 [DOI] [PubMed] [Google Scholar]
- O’Brien PD, Sakowski SA, & Feldman EL (2014). Mouse models of diabetic neuropathy. ILAR J, 54(3), 259–272. doi: 10.1093/ilar/ilt052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, & Trapp BD (2011). Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J Neurosci, 31(20), 7249–7258. doi: 10.1523/jneurosci.0095-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F. (2013). The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med, 34(2–3), 465–484. doi: 10.1016/j.mam.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Pareyson D, Piscosquito G, Moroni I, Salsano E, & Zeviani M. (2013). Peripheral neuropathy in mitochondrial disorders. Lancet Neurol, 12(10), 1011–1024. doi: 10.1016/s1474-4422(13)70158-3 [DOI] [PubMed] [Google Scholar]
- Pareyson D, Saveri P, Sagnelli A, & Piscosquito G. (2015). Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci Lett, 596, 66–77. doi: 10.1016/j.neulet.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Patel MS, Nemeria NS, Furey W, & Jordan F. (2014). The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem, 289(24), 16615–16623. doi: 10.1074/jbc.R114.563148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkurnaz G, Trinidad JC, Wang X, Kong D, & Schwarz TL (2014). Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell, 158(1), 54–68. doi: 10.1016/j.cell.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, & Scorrano L. (2016). Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev Physiol, 78, 505–531. doi: 10.1146/annurev-physiol-021115-105011 [DOI] [PubMed] [Google Scholar]
- Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, . . . Zorzano A. (2005). The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet, 14(11), 1405–1415. doi: 10.1093/hmg/ddi149 [DOI] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, & Saxton WM (2006). Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell, 17(4), 2057–2068. doi: 10.1091/mbc.e05-06-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisciotta C, & Shy ME (2018). Neuropathy. Handb Clin Neurol, 148, 653–665. doi: 10.1016/b978-0-444-64076-5.00042-9 [DOI] [PubMed] [Google Scholar]
- Pitceathly RD, Murphy SM, Cottenie E, Chalasani A, Sweeney MG, Woodward C, . . . Hanna MG (2012). Genetic dysfunction of MT-ATP6 causes axonal Charcot-Marie-Tooth disease. Neurology, 79(11), 1145–1154. doi: 10.1212/WNL.0b013e3182698d8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, . . . Ziegler D. (2017). Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care, 40(1), 136–154. doi: 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M, Tetaud E, Duvezin-Caubet S, Ezkurdia N, Bietenhader M, Rytka J, & di Rago JP (2007). A yeast model of the neurogenic ataxia retinitis pigmentosa (NARP) T8993G mutation in the mitochondrial ATP synthase-6 gene. J Biol Chem, 282(47), 34039–34047. doi: 10.1074/jbc.M703053200 [DOI] [PubMed] [Google Scholar]
- Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, . . . Marchuk DA (2002). A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am J Hum Genet, 71(5), 1189–1194. doi: 10.1086/344210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynier P, Amati-Bonneau P, Verny C, Olichon A, Simard G, Guichet A, . . . Bonneau D. (2004). OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract J Med Genet (Vol. 41, pp. e110). England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieusset J, Fauconnier J, Paillard M, Belaidi E, Tubbs E, Chauvin MA, . . . Ovize M. (2016). Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia, 59(3), 614–623. doi: 10.1007/s00125-015-3829-8 [DOI] [PubMed] [Google Scholar]
- Rinaldi C, Grunseich C, Sevrioukova IF, Schindler A, Horkayne-Szakaly I, Lamperti C, . . . Fischbeck KH (2012). Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am J Hum Genet, 91(6), 1095–1102. doi: 10.1016/j.ajhg.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere JB, Ramalingam S, Lavastre V, Shekarabi M, Holbert S, Lafontaine J, . . . Rouleau GA (2011). KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am J Hum Genet, 89(2), 219–230. doi: 10.1016/j.ajhg.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Kon T, Knight PJ, Sutoh K, & Burgess SA (2013). Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol, 14(11), 713–726. doi: 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, & Hay N. (2006). Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene, 25(34), 4683–4696. doi: 10.1038/sj.onc.1209595 [DOI] [PubMed] [Google Scholar]
- Ross JL, Wallace K, Shuman H, Goldman YE, & Holzbaur EL (2006). Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol, 8(6), 562–570. doi: 10.1038/ncb1421 [DOI] [PubMed] [Google Scholar]
- Rossi MJ, & Pekkurnaz G. (2019). Powerhouse of the mind: mitochondrial plasticity at the synapse. Curr Opin Neurobiol, 57, 149–155. doi: 10.1016/j.conb.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor AM, Polke JM, Houlden H, & Reilly MM (2013). Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol, 9(10), 562–571. doi: 10.1038/nrneurol.2013.179 [DOI] [PubMed] [Google Scholar]
- Rossor AM, Tomaselli PJ, & Reilly MM (2016). Recent advances in the genetic neuropathies. Curr Opin Neurol, 29(5), 537–548. doi: 10.1097/wco.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, & Voeltz GK (2012). Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol, 13(10), 607–625. doi: 10.1038/nrm3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumora AE, Lentz SI, Hinder LM, Jackson SW, Valesano A, Levinson GE, & Feldman EL (2018). Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. FASEB J, 32(1), 195–207. doi: 10.1096/fj.201700206R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumora AE, LoGrasso G, Haidar JA, Dolkowski JJ, Lentz SI, & Feldman EL (2019). Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res, 60(1), 58–70. doi: 10.1194/jlr.M086843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumora AE, LoGrasso G, Hayes JM, Mendelson FE, Tabbey MA, Haidar JA, . . . Feldman EL (2019). The divergent roles of dietary saturated and monounsaturated fatty acids on nerve function in murine models of obesity. J Neurosci. doi: 10.1523/JNEUROSCI.3173-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, & Feldman EL (2002). High glucose induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J, 16, 1738–1748. [DOI] [PubMed] [Google Scholar]
- Ryu SW, Jeong HJ, Choi M, Karbowski M, & Choi C. (2010). Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cell Mol Life Sci, 67(16), 2839–2850. doi: 10.1007/s00018-010-0365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajic M. (2014). Mitochondrial dynamics in peripheral neuropathies. Antioxid Redox Signal, 21(4), 601–620. doi: 10.1089/ars.2013.5822 [DOI] [PubMed] [Google Scholar]
- Saporta MA, Dang V, Volfson D, Zou B, Xie XS, Adebola A, . . . Dimos JT (2015). Axonal Charcot-Marie-Tooth disease patient-derived motor neurons demonstrate disease-specific phenotypes including abnormal electrophysiological properties. Exp Neurol, 263, 190–199. doi: 10.1016/j.expneurol.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, . . . Pennathur S. (2016). Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight, 1(15), e86976. doi: 10.1172/jci.insight.86976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas KM, Lin J, Rajendiran TM, Soni T, Nair V, Hinder LM, . . . Pennathur S. (2018). Shared and distinct lipid-lipid interactions in plasma and affected tissues in a diabetic mouse model. J Lipid Res, 59(2), 173–183. doi: 10.1194/jlr.M077222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton WM, & Hollenbeck PJ (2012). The axonal transport of mitochondria. J Cell Sci, 125(Pt 9), 2095–2104. doi: 10.1242/jcs.053850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH (2014). Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol, 204(7), 1087–1098. doi: 10.1083/jcb.201312123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, . . . Thomas G. (2005). PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. Embo j, 24(4), 717–729. doi: 10.1038/sj.emboj.7600559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivera R, Espinos C, Vilchez JJ, Mas F, Martinez-Rubio D, Chumillas MJ, . . . Sevilla T. (2010). Phenotypical features of the p.R120W mutation in the GDAP1 gene causing autosomal dominant Charcot-Marie-Tooth disease. J Peripher Nerv Syst, 15(4), 334–344. doi: 10.1111/j.1529-8027.2010.00286.x [DOI] [PubMed] [Google Scholar]
- Sivitz WI, & Yorek MA (2010). Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal, 12(4), 537–577. doi: 10.1089/ars.2009.2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, & Youle RJ (2008). Mitochondrial dynamics and apoptosis. Genes Dev, 22(12), 1577–1590. doi: 10.1101/gad.1658508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki Y, Ishido S, . . . Yanagi S. (2013). MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol Cell, 51(1), 20–34. doi: 10.1016/j.molcel.2013.04.023 [DOI] [PubMed] [Google Scholar]
- Sullivan KF, & Cleveland DW (1986). Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A, 83(12), 4327–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, . . . Rizzuto R. (2006). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol, 175(6), 901–911. doi: 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak AG, Herder C, Rathmann W, Brunner EJ, & Kivimaki M. (2012). Prediabetes: a high-risk state for diabetes development. Lancet, 379(9833), 2279–2290. doi: 10.1016/s0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurey P, Tubbs E, Vial G, Jacquemetton J, Bendridi N, Chauvin MA, . . . Rieusset J. (2016). Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver. J Mol Cell Biol, 8(2), 129–143. doi: 10.1093/jmcb/mjw004 [DOI] [PubMed] [Google Scholar]
- Thorburn DR, Rahman J, & Rahman S. (1993). Mitochondrial DNA-Associated Leigh Syndrome and NARP. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, & Amemiya A. (Eds.), GeneReviews((R)). Seattle (WA): University of Washington, Seattle: [PubMed] [Google Scholar]
- University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, . . . Engle EC (2010). Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell, 140(1), 74–87. doi: 10.1016/j.cell.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tradewell ML, Durham HD, Mushynski WE, & Gentil BJ (2009). Mitochondrial and axonal abnormalities precede disruption of the neurofilament network in a model of charcot-marie-tooth disease type 2E and are prevented by heat shock proteins in a mutant-specific fashion. J Neuropathol Exp Neurol, 68(6), 642–652. doi: 10.1097/NEN.0b013e3181a5deeb [DOI] [PubMed] [Google Scholar]
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, . . . Yoshikawa S. (1996). The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science, 272(5265), 1136–1144. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, . . . Hoogenraad CC (2013). TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron, 77(3), 485–502. doi: 10.1016/j.neuron.2012.11.027 [DOI] [PubMed] [Google Scholar]
- Vas PRJ, & Edmonds ME (2016). Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol, 4(9), 723–725. doi: 10.1016/s2213-8587(16)30063-8 [DOI] [PubMed] [Google Scholar]
- Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, . . . Feldman EL. (2010). Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol, 120(4), 477–489. doi: 10.1007/s00401-010-0697-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, & Feldman EL (2005). Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J, 19(6), 638–640. [DOI] [PubMed] [Google Scholar]
- Vollrath JT, Sechi A, Dreser A, Katona I, Wiemuth D, Vervoorts J, . . . Goswami A. (2014). Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis, 5, e1290. doi: 10.1038/cddis.2014.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, & Barres BA (2012). Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol, 196(1), 7–18. doi: 10.1083/jcb.201108111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, & Leonard JV (2007). A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med, 356(17), 1736–1741. doi: 10.1056/NEJMoa064436 [DOI] [PubMed] [Google Scholar]
- Weedon MN, Hastings R, Caswell R, Xie W, Paszkiewicz K, Antoniadi T, . . . Ellard S. (2011). Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet, 89(2), 308–312. doi: 10.1016/j.ajhg.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T, Davies JR, Guschina IA, Ball DJ, Davies JS, Davies VJ, . . . Votruba, M. (2012). Opa3, a novel regulator of mitochondrial function, controls thermogenesis and abdominal fat mass in a mouse model for Costeff syndrome. Hum Mol Genet, 21(22), 4836–4844. doi: 10.1093/hmg/dds315 [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu H, Gu M, Luo Q, Ding J, Yao Y, . . . Wang Z. (2013). DHTKD1 is essential for mitochondrial biogenesis and function maintenance. FEBS Lett, 587(21), 3587–3592. doi: 10.1016/j.febslet.2013.08.047 [DOI] [PubMed] [Google Scholar]
- Xu WY, Gu MM, Sun LH, Guo WT, Zhu HB, Ma JF, . . . Wang ZG. (2012). A nonsense mutation in DHTKD1 causes Charcot-Marie-Tooth disease type 2 in a large Chinese pedigree. Am J Hum Genet, 91(6), 1088–1094. doi: 10.1016/j.ajhg.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Coppey LJ, Kardon RH, & Yorek MA (2017). Early vs. late intervention of high fat/low dose streptozotocin treated C57Bl/6J mice with enalapril, alpha-lipoic acid, menhaden oil or their combination: Effect on diabetic neuropathy related endpoints. Neuropharmacology, 116, 122–131. doi: 10.1016/j.neuropharm.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SB, & Pekkurnaz G. (2018). Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J Mol Biol, 430(21), 3922–3941. doi: 10.1016/j.jmb.2018.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S, . . . Carelli V. (2008). OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain, 131(Pt 2), 352–367. doi: 10.1093/brain/awm335 [DOI] [PubMed] [Google Scholar]
- Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, . . . Hirokawa N. (2001). Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell, 105(5), 587–597. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang Y, Xu H, Fu Y, Qian T, Bo D, . . . Chen XJ. (2016). Dync1h1 Mutation Causes Proprioceptive Sensory Neuron Loss and Impaired Retrograde Axonal Transport of Dorsal Root Ganglion Neurons. CNS Neurosci Ther, 22(7), 593–601. doi: 10.1111/cns.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Kennerson ML, Walizada G, Zuchner S, Vance JM, & Nicholson GA (2005). Charcot-Marie-Tooth with pyramidal signs is genetically heterogeneous: families with and without MFN2 mutations. Neurology, 65(3), 496–497. doi: 10.1212/01.wnl.0000171345.62270.29 [DOI] [PubMed] [Google Scholar]
- Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, . . . Chen W. (2012). Quantitative imaging of energy expenditure in human brain. Neuroimage, 60(4), 2107–2117. doi: 10.1016/j.neuroimage.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW (2014). Mechanisms of diabetic neuron damage: Molecular pathways. Handb Clin Neurol, 126, 379–399. doi: 10.1016/b978-0-444-53480-4.00028-x [DOI] [PubMed] [Google Scholar]
- Zuchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, . . . Vance, J. M. (2006). Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol, 59(2), 276–281. doi: 10.1002/ana.20797 [DOI] [PubMed] [Google Scholar]