Abstract
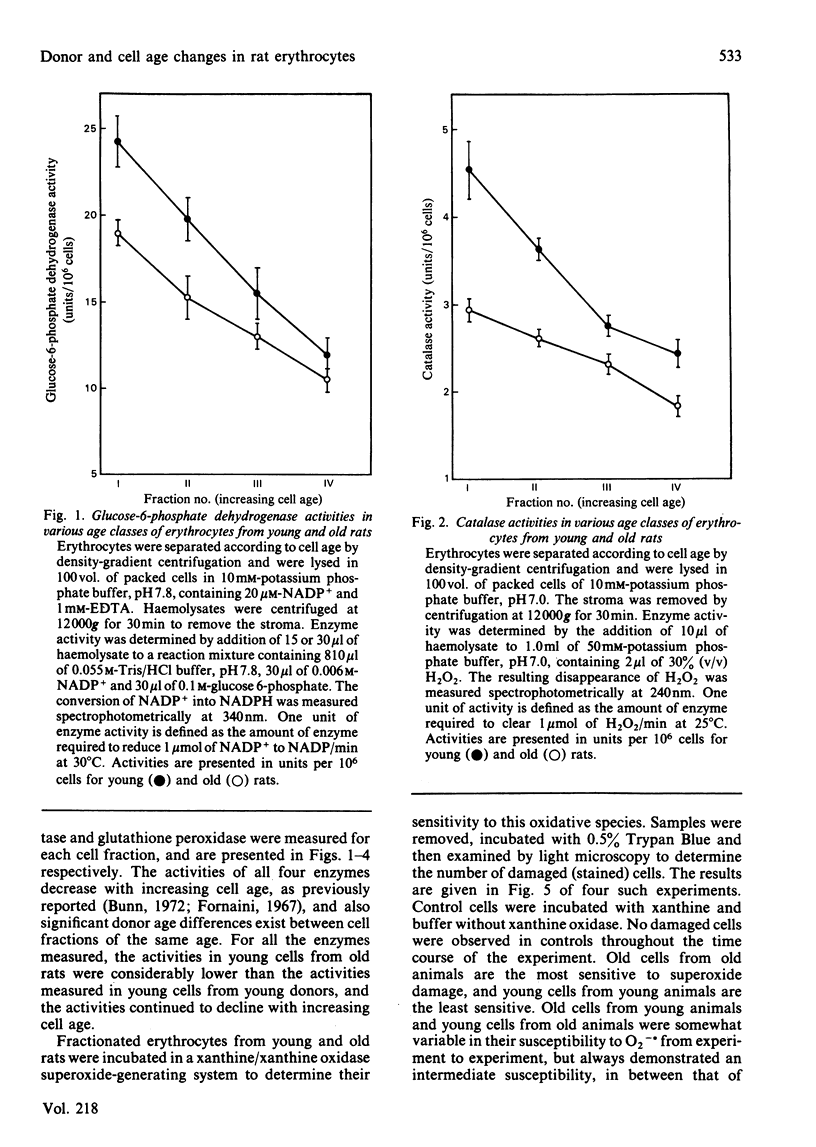
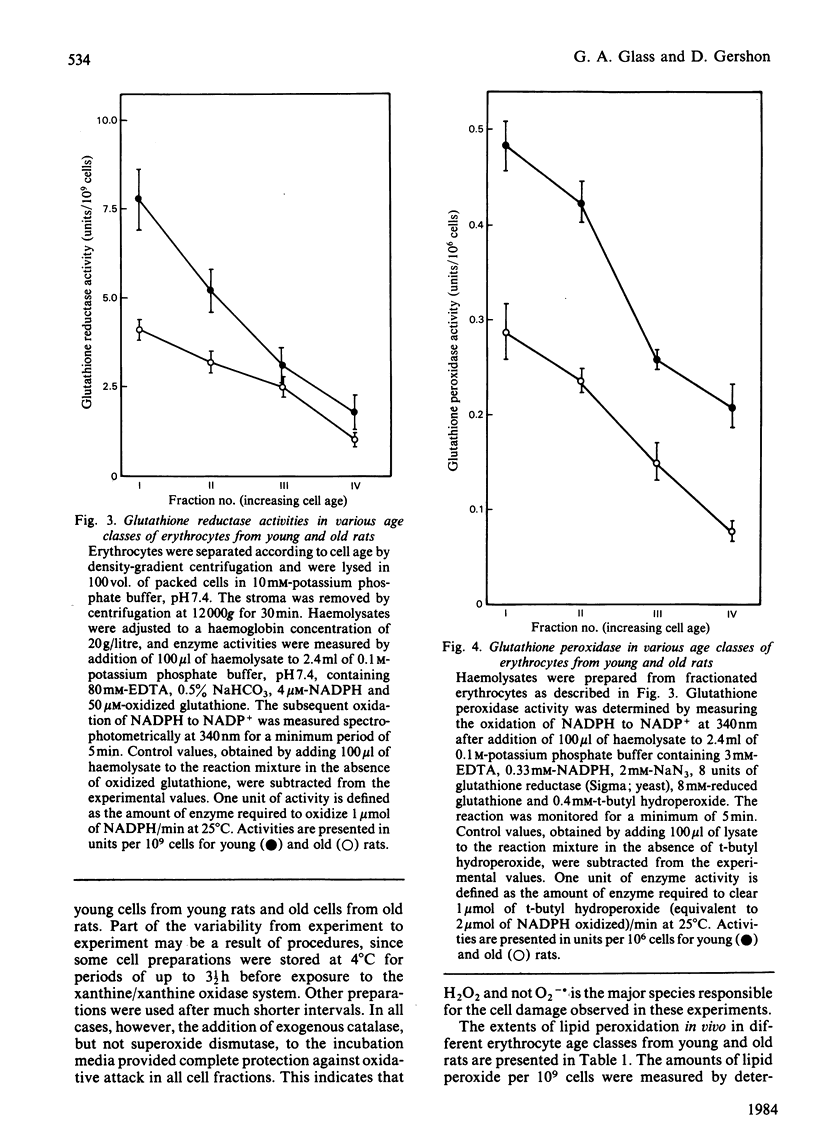
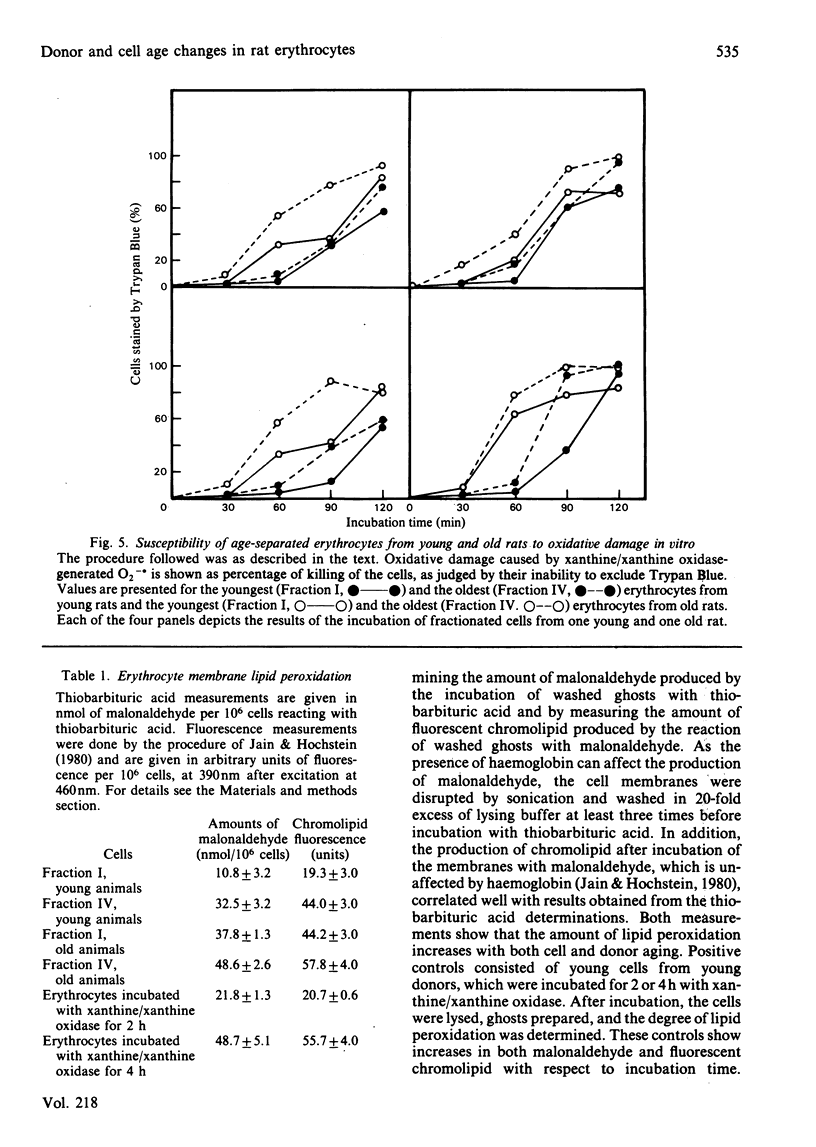
Erythrocytes from young and old rats were separated into four age fractions by density-gradient centrifugation. The specific activities per cell were determined for glucose-6-phosphate dehydrogenase (EC 1.1.1.49), glutathione peroxidase (EC 1.11.1.9), glutathione reductase (EC 1.6.4.2) and catalase (EC 1.11.1.6). Decreased specific activities were observed with increasing cell age for all four enzymes in both young and old animals. In addition, significant differences in the activities of these enzymes were observed between cells of the same age fraction from young and old donors. Susceptibility of fractionated erythrocytes to oxidative attack in vitro generated by incubation with xanthine/xanthine oxidase increased with both cell and animal age. The amount of membrane-lipid peroxidation also increased with cell and animal aging, as measured by both thiobarbituric acid and fluorescent chromolipid assays. Increases of 2-3-fold in the contents of lipid peroxides were observed between the youngest and oldest age fractions of young rats. Lipid peroxide contents in young cells of old animals were equal to those in old erythrocytes from young rats and increased by 30% with cell aging in the old donors. These results suggest that the extent of enzymic protection against oxidative and peroxidative damage decreases with erythrocyte aging. More importantly, enzymic protection in cells of old rats is considerably decreased already in the early stages of their lifespan.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Taylor J. F., Lang C. A. Influence of mouse age and erythrocyte age on glutathione metabolism. Biochem J. 1978 Sep 15;174(3):819–825. doi: 10.1042/bj1740819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BORUN E. R., FIGUEROA W. G., PERRY S. M. The distribution of Fe59 tagged human erythrocytes in centrifuged specimens as a function of cell age. J Clin Invest. 1957 May;36(5):676–679. doi: 10.1172/JCI103468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F. Erythocyte destruction and hemoglobin catabolism. Semin Hematol. 1972 Jan;9(1):3–17. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Detraglia M., Cook F. B., Stasiw D. M., Cerny L. C. Erythrocyte fragility in aging. Biochim Biophys Acta. 1974 Apr 29;345(2):213–219. doi: 10.1016/0005-2736(74)90259-4. [DOI] [PubMed] [Google Scholar]
- Glass G. A., Gershon D. Enzymatic changes in rat erythrocytes with increasing cell and donor age: loss of superoxide dismutase activity associated with increases in catalytically defective forms. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1245–1253. doi: 10.1016/0006-291x(81)90256-4. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Ohrloff C., Lange G., Hockwin O. Postsynthetic changes of glutathione peroxidase (EC 1.11.1.9) and glutathione reductase (EC 1.6.4.2) in the ageing bovine lens. Mech Ageing Dev. 1980 Nov-Dec;14(3-4):453–458. doi: 10.1016/0047-6374(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and hemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. Dependence on glucose metabolism and hemoglobin status. Biochim Biophys Acta. 1981 Dec 4;678(2):230–237. doi: 10.1016/0304-4165(81)90211-7. [DOI] [PubMed] [Google Scholar]


