Abstract
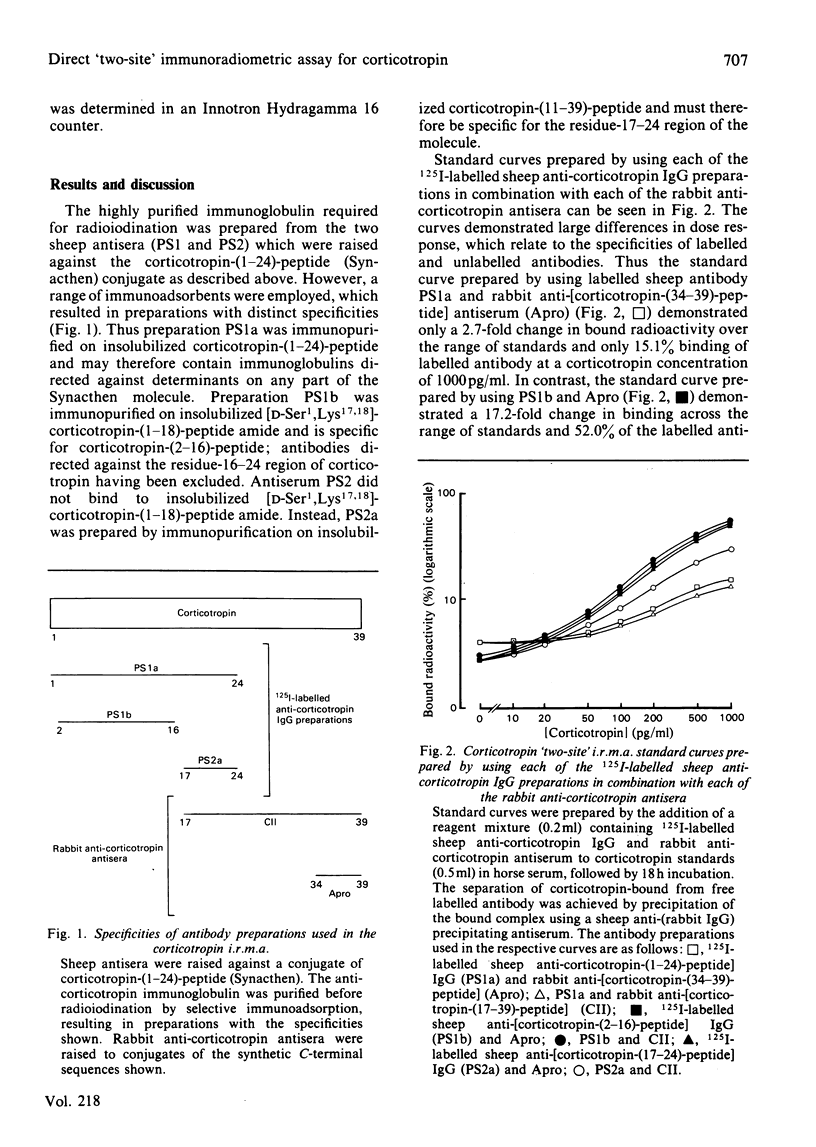
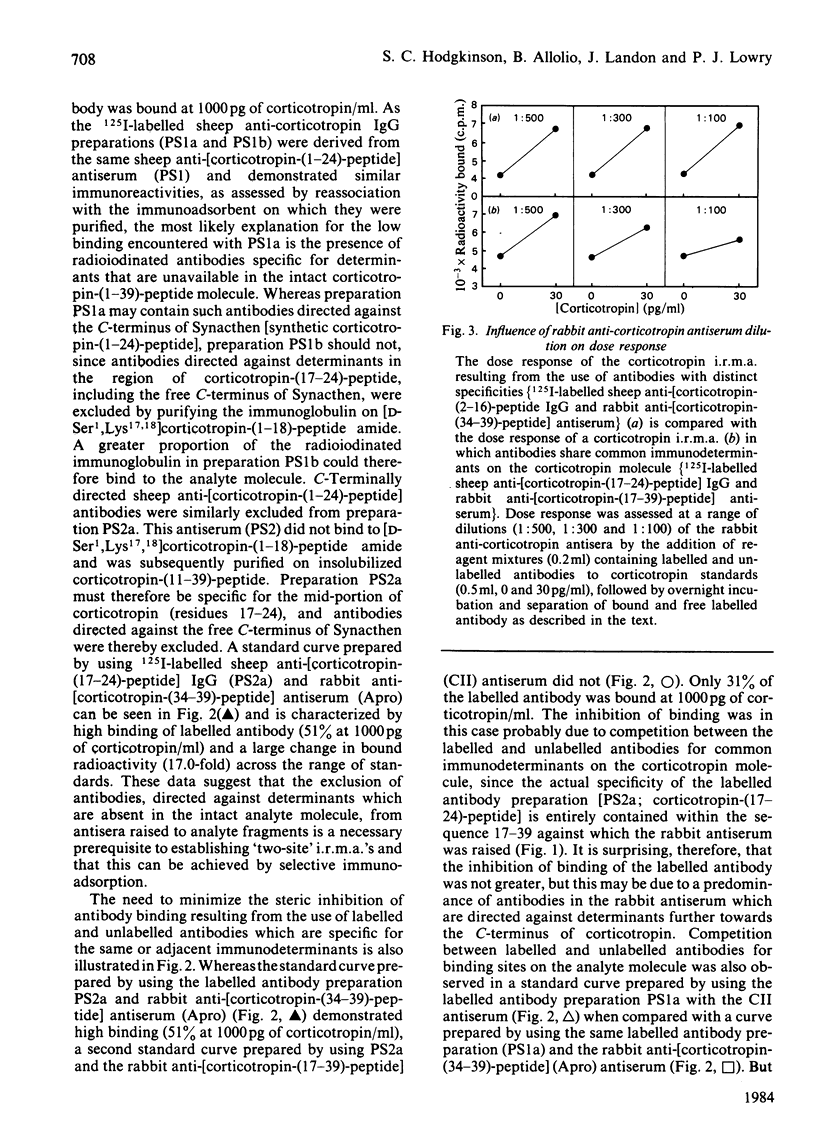
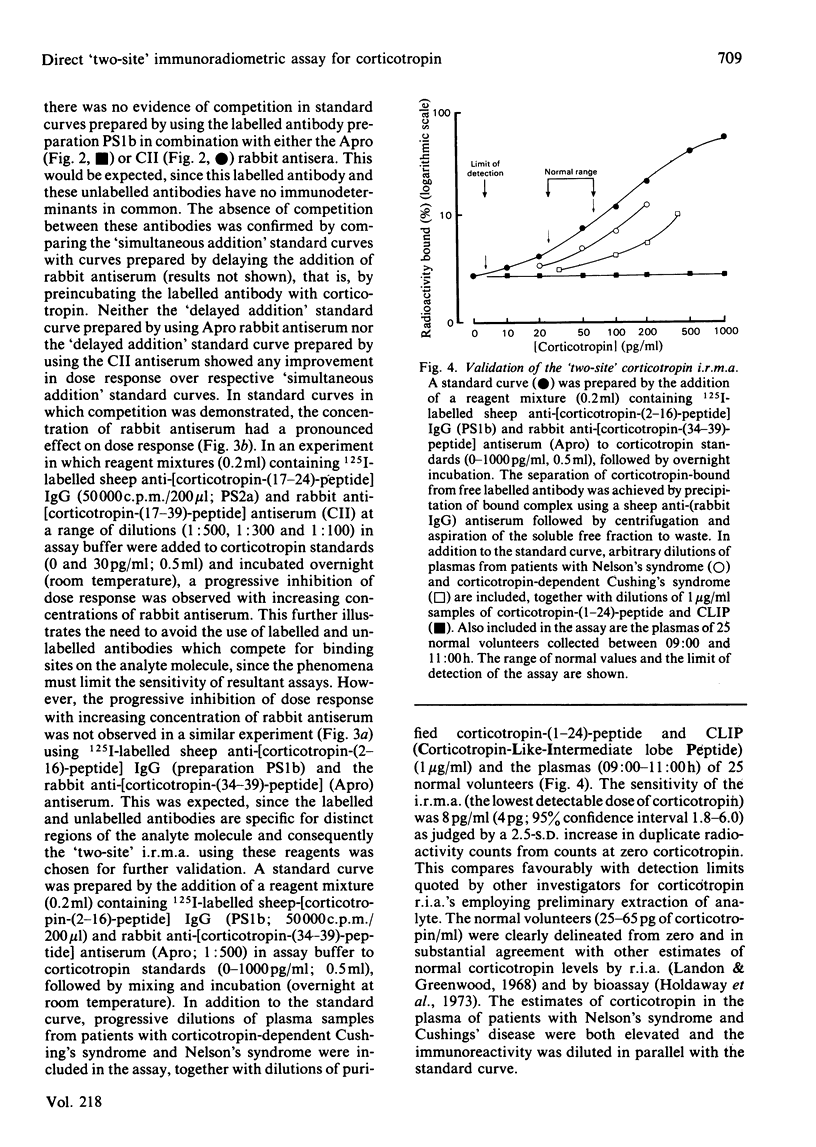
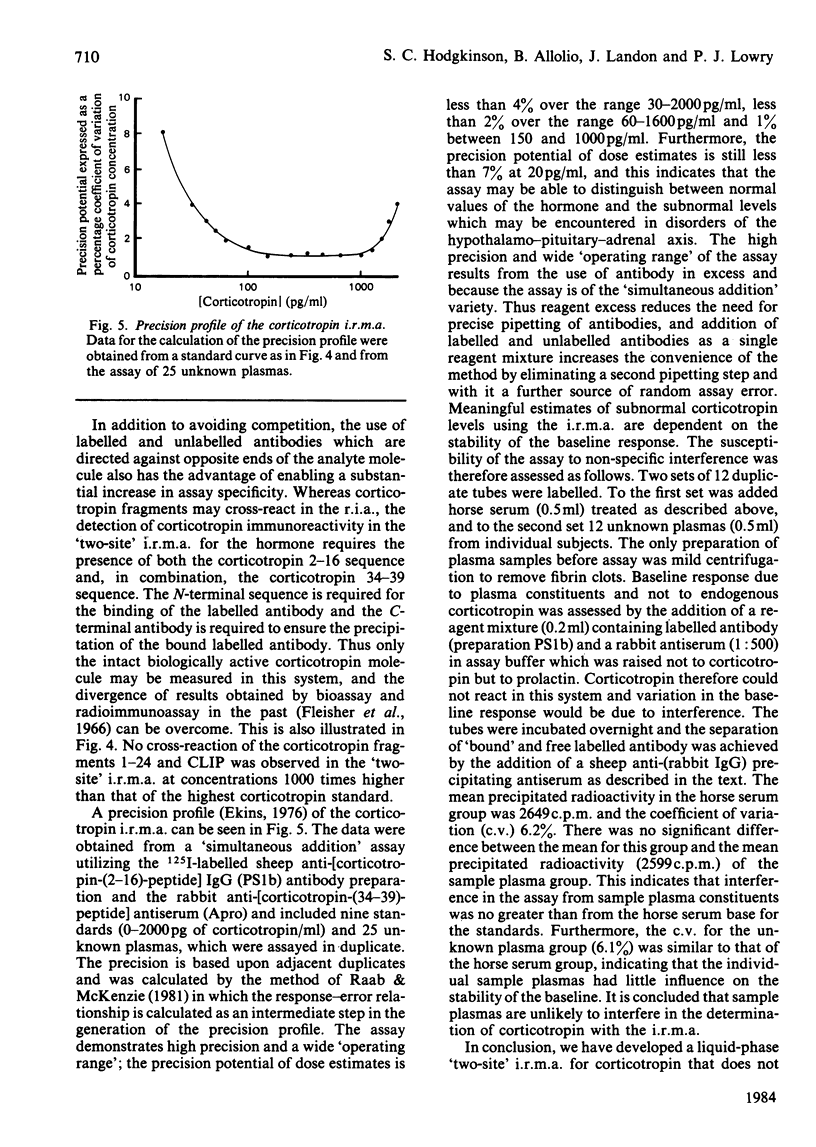
The development of a 'two-site' immunoradiometric assay (i.r.m.a.) for the direct estimation of human corticotropin-(1-39)-peptide in plasma is described. The assay is based on the simultaneous addition of 125I-labelled sheep anti-(N-terminal corticotropin) IgG (immunoglobulin G) antibodies and rabbit anti-(C-terminal corticotropin) antiserum to standards and unknowns (0.5 ml) followed by 18h incubation. The use of solid-phase reagents was avoided in order to minimize non-specific effects and the time required for reactants to reach equilibrium. Instead, the separation of corticotropin-bound from free labelled antibody is achieved by the addition of sheep anti-(rabbit IgG) antiserum, which precipitates bound labelled antibody by complex-formation with rabbit anti-corticotropin antibodies, which are also hormone-bound. Several 125I-labelled sheep anti-(N-terminal corticotropin) IgG preparations were assessed in the i.r.m.a. Although each was derived from antisera raised to a thyroglobulin conjugate of synthetic corticotropin-(1-24)-peptide (Synacthen), purification of immunoglobulins before iodination by selective immunoadsorption resulted in preparations with distinct specificities which demonstrated marked differences in binding to intact human corticotropin-(1-39)-peptide. These preparations are compared in combination with two rabbit anti-(C-terminal corticotropin) antisera. A 'two-site' assay based on the use of 125I-labelled sheep anti-[ corticotropin-(2-16)-peptide] IgG and rabbit anti-[corticotropin-(34-39)-peptide] antiserum was optimized, since steric inhibition of antibody binding was avoided with this combination and because the measurement of only intact human corticotropin-(1-39)-peptide and not fragments was assured by the use of terminal antibodies. This i.r.m.a. is characterized by rapid equilibration of reactants, a wide 'operating range' (the precision of dose estimates was less than 4% over the range 30-2200 pg/ml) and high sensitivity [8 pg of corticotropin/ml (95% confidence interval 3.7-12.0) (4 pg minimal detectable mass) can be detected directly in plasma].
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVEN D. W., ESPINER E. A., HART D. S. THE SUPPRESSION OF CORTISOL SECRETION BY STEROIDS, AND RESPONSE TO CORTICOTROPHIN, IN SHEEP WITH ADRENAL TRANSPLANTS. J Physiol. 1964 Jun;171:216–230. doi: 10.1113/jphysiol.1964.sp007373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Radioimmunoassay of ACTH in plasma. J Clin Invest. 1968 Dec;47(12):2725–2751. doi: 10.1172/JCI105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. A. Application of the coated charcoal separation method to the radio-immunoassay of plasma corticotrophin. J Endocrinol. 1968 Aug;41(4):499–508. doi: 10.1677/joe.0.0410499. [DOI] [PubMed] [Google Scholar]
- Fleischer N., Givens J. R., Abe K., Nicholson W. E., Liddle G. W. Studies of ACTH antibodies and their reactions with inactive analogues of ACTH. Endocrinology. 1966 May;78(5):1067–1075. doi: 10.1210/endo-78-5-1067. [DOI] [PubMed] [Google Scholar]
- Hodgkinson S. C., Lowry P. J. Selective elution of immunoadsorbed anti-(human prolactin) immunoglobulins with enhanced immunochemical properties. Biochem J. 1982 Sep 1;205(3):535–541. doi: 10.1042/bj2050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway I. M., Rees L. H., Landon J. Circulating corticotrophin levels in severe hypopituitarism and in the neonate. Lancet. 1973 Nov 24;2(7839):1170–1171. doi: 10.1016/s0140-6736(73)92938-3. [DOI] [PubMed] [Google Scholar]
- Hunter W. M., Budd P. S. Immunoradiometric versus radioimmunoassay: a comparison using alpha-fetoprotein as the model analyte. J Immunol Methods. 1981;45(3):255–273. doi: 10.1016/0022-1759(81)90304-5. [DOI] [PubMed] [Google Scholar]
- Imura H., Sparks L. L., Grodsky G. M., Forsham P. H. Immunologic studies of adrenocorticotropic hormone (ACTH): dissociation of biologic and immunologic activities. J Clin Endocrinol Metab. 1965 Oct;25(10):1361–1369. doi: 10.1210/jcem-25-10-1361. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsen A. R., McIntyre H. B., Odell W. D. Adrenocorticotropin measurement by competitive binding receptor assay. J Clin Endocrinol Metab. 1972 Apr;34(4):684–689. doi: 10.1210/jcem-34-4-684. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., GLICK S. M., ROTH J., BERSON S. A. RADIOIMMUNOASSAY OF HUMAN PLASMA ACTH. J Clin Endocrinol Metab. 1964 Nov;24:1219–1225. doi: 10.1210/jcem-24-11-1219. [DOI] [PubMed] [Google Scholar]


