Abstract
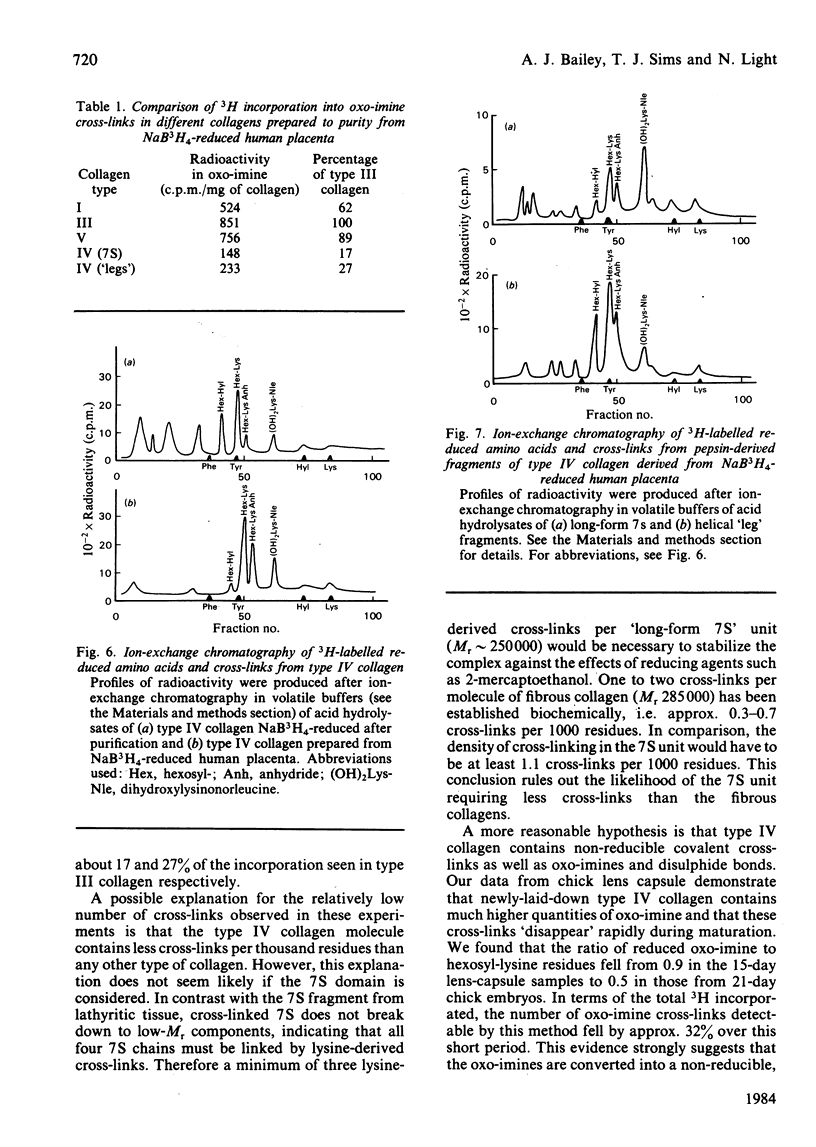
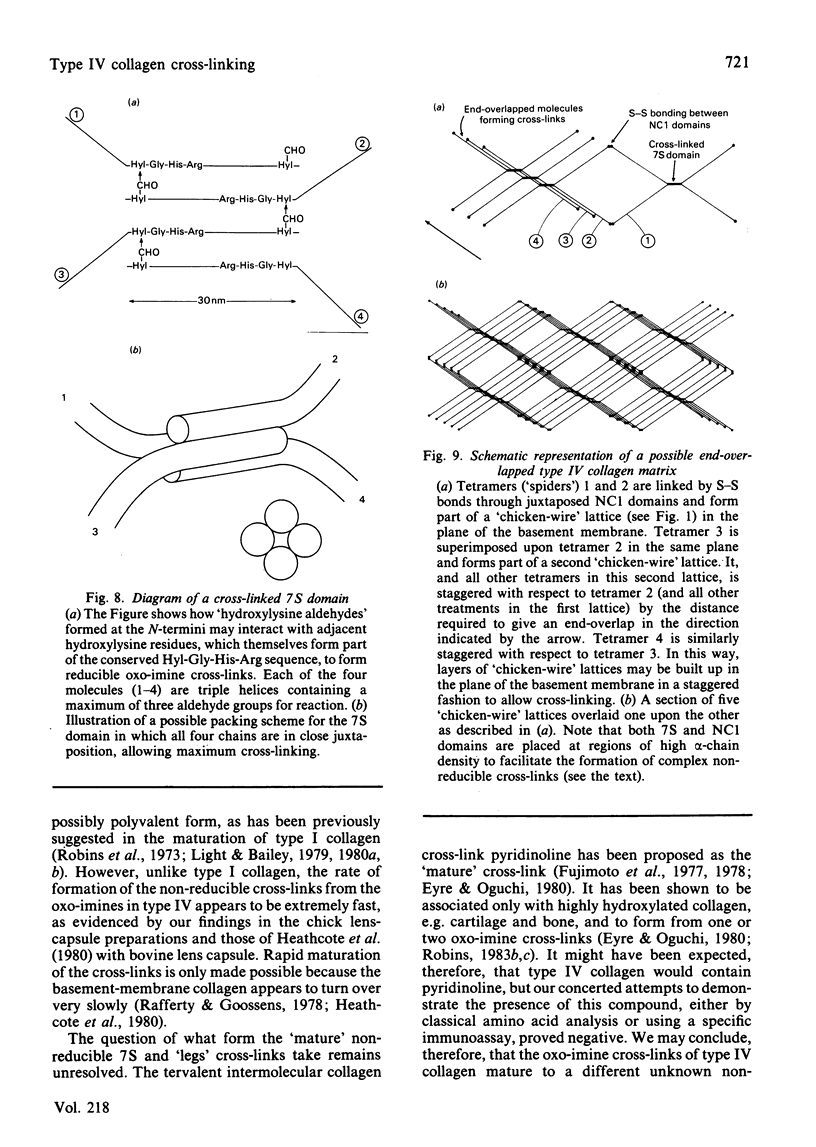
Type IV collagen could not be extracted from human placenta using 6M-urea containing 10mM-dithiothreitol, indicating that the type IV molecule is stabilized within the basement membrane by covalent cross-links. However, various forms of type IV collagen molecule were extractable by means of increasingly severe proteolytic conditions. Type IV collagen tetramers ('spiders') lacking only the C-terminal globular region (NC1) were further digested to the 'long-form' 7S fragment and an assortment of helical rod-like molecules derived from the 'leg' region. These molecules were separated by salt fractionation and examined by rotary-shadowing electron microscopy. Isolation of these fractions from placenta which had been reduced with NaB3H4 revealed that both the 7S (N-terminal) and C-terminal regions contained significant proportions of reducible lysine-derived cross-links. These cross-links were shown to be exclusively the stable oxo-imine hydroxylysino-5-oxonorleucine. The number of the cross-links per molecule was significantly lower than might be expected in order to fully stabilize the molecule. These results suggest that the keto-imine cross-links in type IV collagen have been stabilized in part by conversion into an unknown non-reducible form, although a sensitive immunoassay failed to show the presence of any pyridinoline. Comparison with the fibrous collagen from placenta suggested that the rate of this conversion is much greater in basement-membrane collagen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Sims T. J., Duance V. C., Light N. D. Partial characterization of a second basement membrane collagen in human placenta. Evidence for the existence of two type IV collagen molecules. FEBS Lett. 1979 Mar 15;99(2):361–366. doi: 10.1016/0014-5793(79)80992-8. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Stuart J. M., Seyer J. M., Risteli J., Timpl R., Kang A. H. Type IV collagens' isolation and characterization of 7S collagen from human kidney, liver and lung. Coll Relat Res. 1981 Nov;1(6):549–556. doi: 10.1016/s0174-173x(81)80036-2. [DOI] [PubMed] [Google Scholar]
- Duncan K. G., Fessler L. I., Bächinger H. P., Fessler J. H. Procollagen IV. Association to tetramers. J Biol Chem. 1983 May 10;258(9):5869–5877. [PubMed] [Google Scholar]
- Elsden D. F., Light N. D., Bailey A. J. An investigation of pyridinoline, and putative collagen cross-link. Biochem J. 1980 Feb 1;185(2):531–534. doi: 10.1042/bj1850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Oguchi H. The hydroxypyridinium crosslinks of skeletal collagens: their measurement, properties and a proposed pathway of formation. Biochem Biophys Res Commun. 1980 Jan 29;92(2):403–410. doi: 10.1016/0006-291x(80)90347-2. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Isolation and characterization of a fluorescent material in bovine achilles tendon collagen. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1124–1129. doi: 10.1016/0006-291x(77)90972-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Moriguchi T., Ishida T., Hayashi H. The structure of pyridinoline, a collagen crosslink. Biochem Biophys Res Commun. 1978 Sep 14;84(1):52–57. doi: 10.1016/0006-291x(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Glanville R. W., Rauter A., Fietzek P. P. Isolation and characterization of a native placental basement-membrane collagen and its component alpha chains. Eur J Biochem. 1979 Apr 2;95(2):383–389. doi: 10.1111/j.1432-1033.1979.tb12976.x. [DOI] [PubMed] [Google Scholar]
- Heathcote J. G., Bailey A. J., Grant M. E. Studies on the assembly of the rat lens capsule. Biosynthesis of a cross-linked collagenous component of high molecular weight. Biochem J. 1980 Aug 15;190(2):229–237. doi: 10.1042/bj1900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kühn K., Wiedemann H., Timpl R., Risteli J., Dieringer H., Voss T., Glanville R. W. Macromolecular structure of basement membrane collagens. FEBS Lett. 1981 Mar 9;125(1):123–128. doi: 10.1016/0014-5793(81)81012-5. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Changes in crosslinking during aging in bovine tendon collagen. FEBS Lett. 1979 Jan 1;97(1):183–188. doi: 10.1016/0014-5793(79)80080-0. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Polymeric C-terminal cross-linked material from type-I collagen. A modified method for purification, anomalous behaviour on gel filtration, molecular weight estimation, carbohydrate content and lipid content. Biochem J. 1980 Jul 1;189(1):111–124. doi: 10.1042/bj1890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. The chemistry of the collagen cross-links. Purification and characterization of cross-linked polymeric peptide material from mature collagen containing unknown amino acids. Biochem J. 1980 Feb 1;185(2):373–381. doi: 10.1042/bj1850373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. An enzyme-linked immunoassay for the collagen cross-link pyridinoline. Biochem J. 1982 Dec 1;207(3):617–620. doi: 10.1042/bj2070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. Cross-linking of collagen. Isolation, structural characterization and glycosylation of pyridinoline. Biochem J. 1983 Oct 1;215(1):167–173. doi: 10.1042/bj2150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Duncan A. Cross-linking of collagen. Location of pyridinoline in bovine articular cartilage at two sites of the molecule. Biochem J. 1983 Oct 1;215(1):175–182. doi: 10.1042/bj2150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B. C., Bailey A. J. Molecular weight heterogeneity of the alpha-chain sub-units of collagen. Biochem Biophys Res Commun. 1971 Apr 16;43(2):340–345. doi: 10.1016/0006-291x(71)90758-3. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Kefalides N. A. Collagen crosslinks: occurrence in basement membrane collagens. Biochem Biophys Res Commun. 1973 Apr 2;51(3):775–780. doi: 10.1016/0006-291x(73)91382-x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Wu V. Y., Cogen M. P. Reducible cross-links in human glomerular basement membrane. Biochem Biophys Res Commun. 1982 Feb 11;104(3):911–915. doi: 10.1016/0006-291x(82)91335-3. [DOI] [PubMed] [Google Scholar]
- le Pape A., Guitton J. D., Muh J. P. Modification of glomerular basement membrane cross-links in experimental diabetic rats. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1214–1221. doi: 10.1016/0006-291x(81)91953-7. [DOI] [PubMed] [Google Scholar]