Abstract
The relationship of lactate metabolism to renal function was studied in the isolated perfused rat kidney. A new radioisotopic method has been developed that enables the simultaneous measurement of lactate production and consumption in the presence of physiological concentrations of both lactate and glucose. In kidneys from fed rats, when glucose was absent, lactate production was only 12 mumol/h per g dry wt, and in kidneys from starved rats there was no lactate production, indicating that neither the phosphoenolpyruvate/pyruvate substrate cycle nor other analogous cycles for the recycling of lactate carbon are operating in the intact kidney cortex. Lactate production from glucose occurred at a high rate, at the same time as lactate consumption, demonstrating that lactate recycling between renal cortex and medulla can occur in the intact kidney. Lactate production from glucose correlated with glomerular filtration rate (P less than 0.001), urine flow rate (P less than 0.01) and sodium reabsorption (P less than 0.05). There was significant basal lactate production at zero glomerular filtration rate. Lactate consumption was not correlated with any renal function. When Na+ reabsorption was inhibited with the diuretic frusemide, or when filtration was entirely prevented (the 'non'-filtering kidney'), lactate production was decreased by 39% and 50% respectively. Basal lactate production determined in this way was the same as that calculated above by linear regression. Prevention of filtration, but not the addition of frusemide, significantly inhibited lactate consumption. It is concluded that glycolysis is required for medullary Na+ transport, and that some different transport function(s) require lactate oxidation.
Full text
PDF


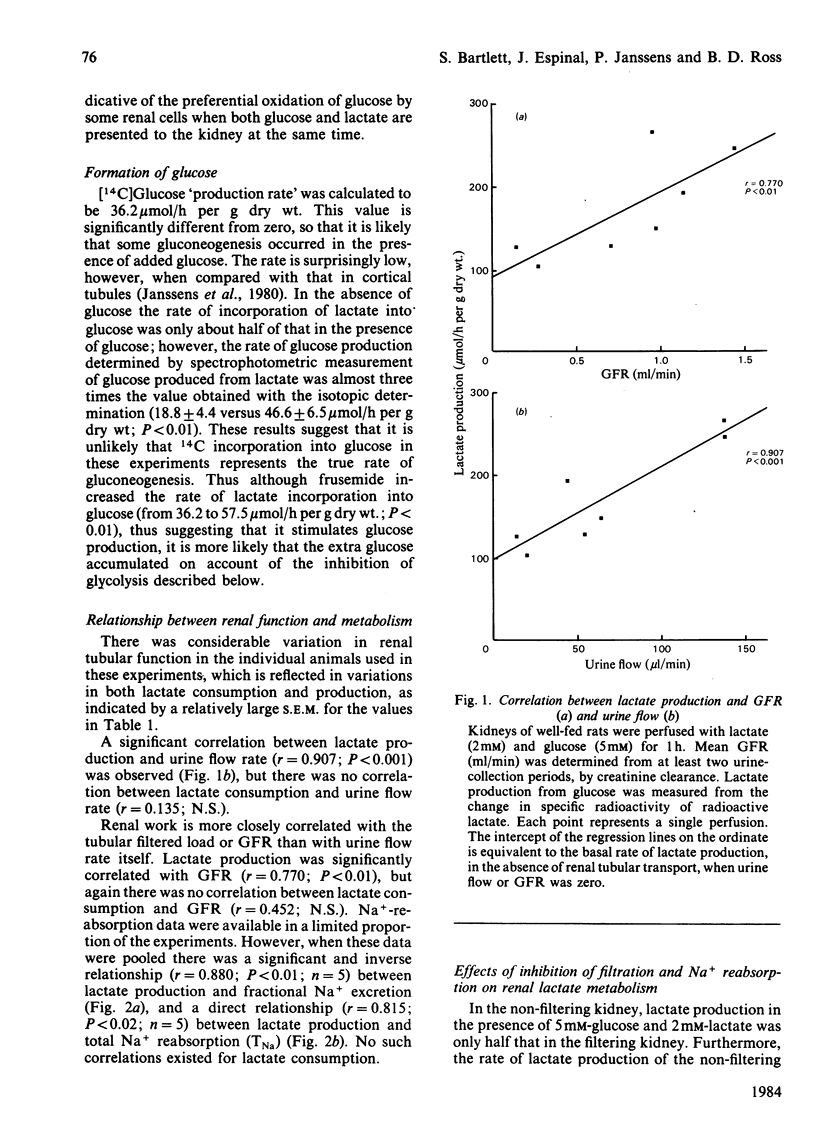
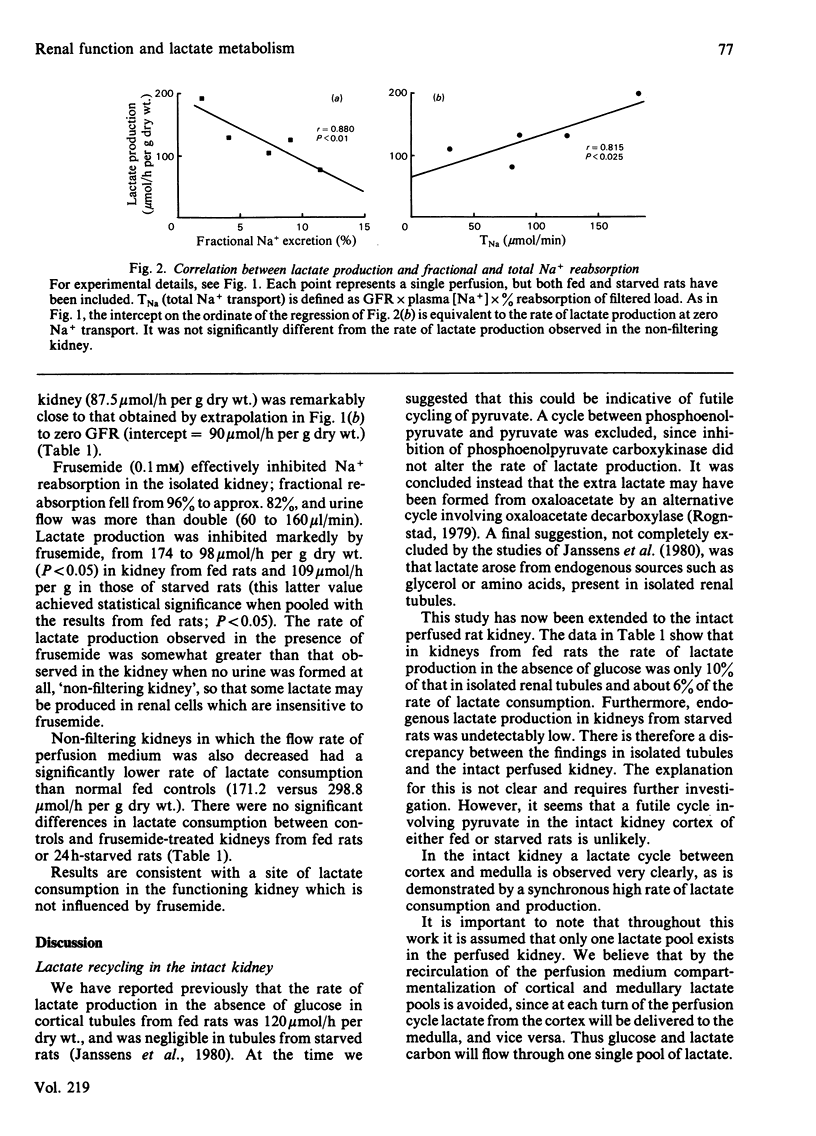

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines A. D., Ross B. D. Nonoxidative glucose metabolism a prerequisite for formation of dilute urine. Am J Physiol. 1982 May;242(5):F491–F498. doi: 10.1152/ajprenal.1982.242.5.F491. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Merkens L. S., Peterson O. W. Relation of Na+ reabsorption to utilization of O2 and lactate in the perfused rat kidney. Am J Physiol. 1980 May;238(5):F415–F427. doi: 10.1152/ajprenal.1980.238.5.F415. [DOI] [PubMed] [Google Scholar]
- DEETJEN P., KRAMER K. [Sodium-re-absorption and oxygen consumption by the kidneys]. Klin Wochenschr. 1960 Jul 15;38:680–680. doi: 10.1007/BF01486946. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H. A rapid method for isolating glucose metabolites involved in substrate cycling. Anal Biochem. 1980 Dec;109(2):443–448. doi: 10.1016/0003-2697(80)90675-2. [DOI] [PubMed] [Google Scholar]
- Janssens P., Hems R., Ross B. The metabolic fate of lactate in renal cortical tubules. Biochem J. 1980 Jul 15;190(1):27–37. doi: 10.1042/bj1900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W. Der architektonische und funktionelle Aufbau der Rattenniere. Z Zellforsch Mikrosk Anat. 1967;82(4):495–535. [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R. Pyruvate cycling involving possible oxaloacetate decarboxylase activity. Biochim Biophys Acta. 1979 Aug 22;586(2):242–249. doi: 10.1016/0304-4165(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Vinay P., Mapes J. P., Krebs H. A. Fate of glutamine carbon in renal metabolism. Am J Physiol. 1978 Feb;234(2):F123–F129. doi: 10.1152/ajprenal.1978.234.2.F123. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]


