Abstract
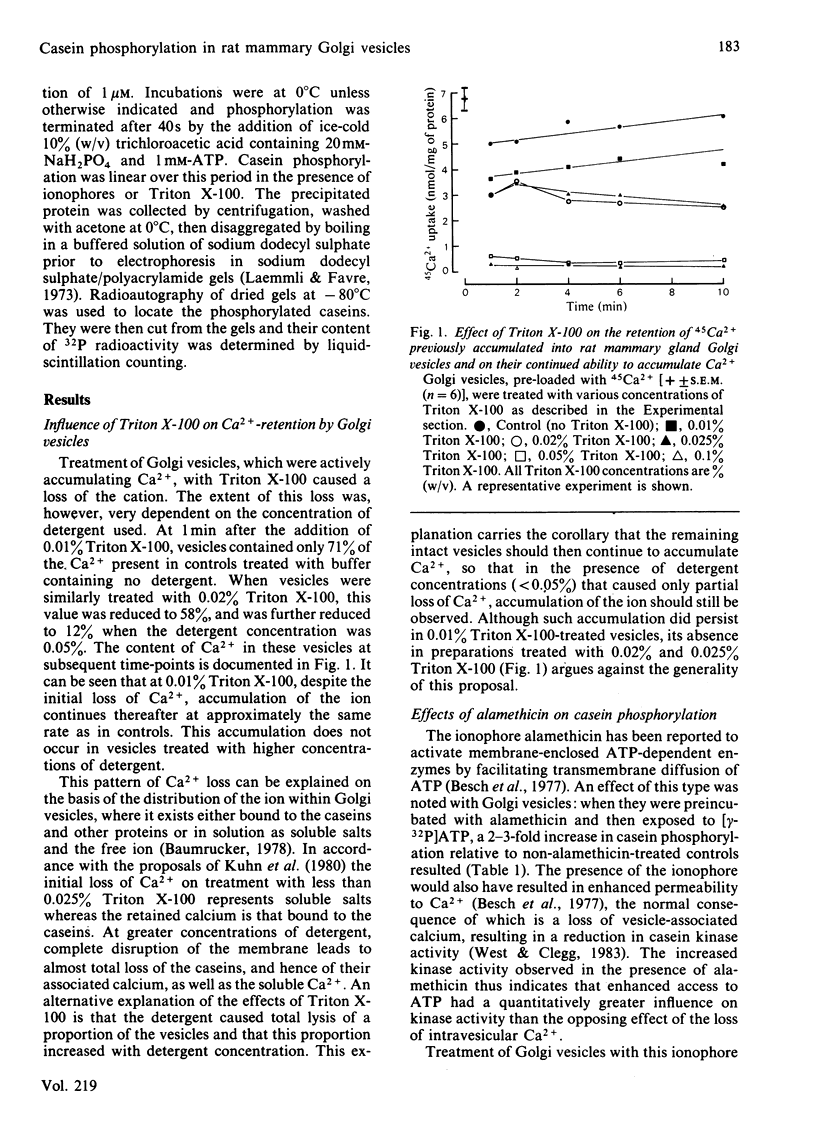
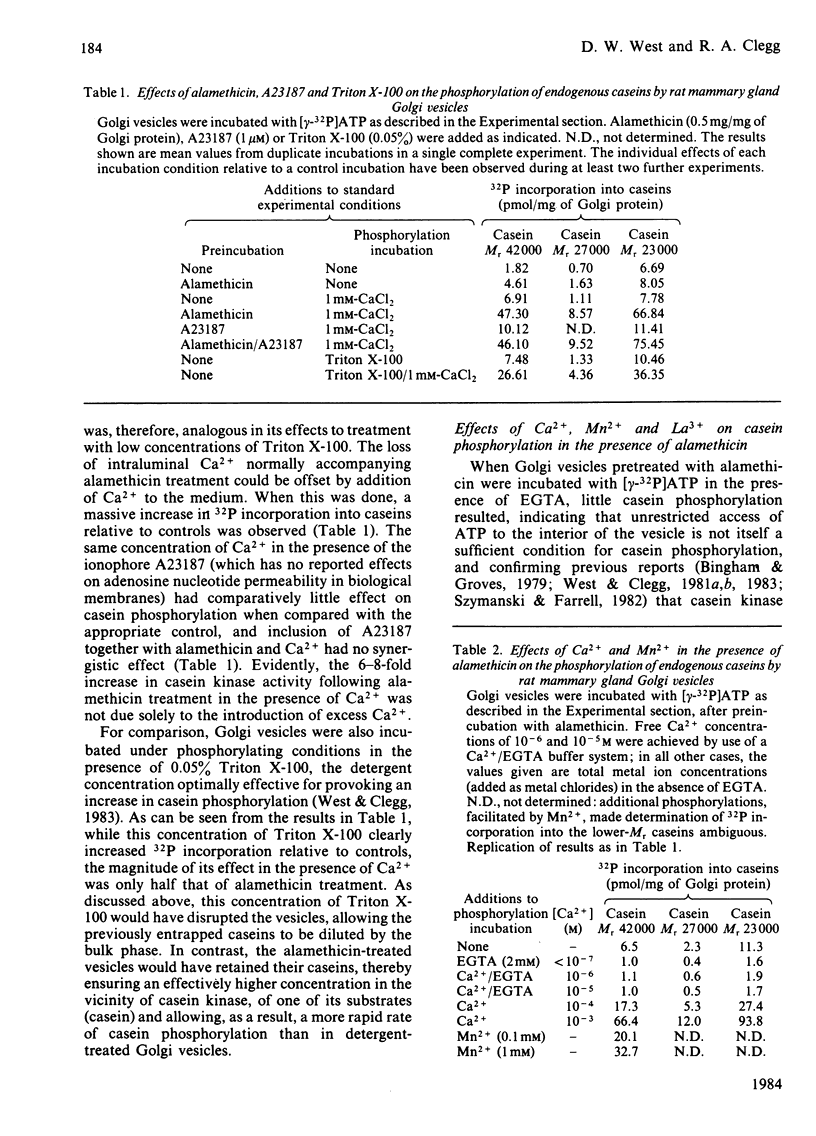
A Golgi vesicle-enriched preparation from mammary tissue of lactating rats has been used to investigate the phosphorylation of caseins in vitro. Casein kinase, together with its casein substrates, is enclosed within the lumen of Golgi membrane vesicles and has a requirement for Ca2+ and ATP. The permeability characteristics of the Golgi membrane to ATP and Ca2+ therefore have a possible regulatory influence on casein kinase activity. This influence has been investigated by alteration of the permeability characteristics by using several agents having differing degrees of selectivity. The ionophore A23187, which permits loss of Ca2+ from the vesicles, caused a decrease in casein phosphorylation which could be reversed by externally supplied Ca2+. Alamethicin, an ionophore that creates larger transmembrane channels, caused an increase in casein phosphorylation. This increase showed a requirement for divalent metal ions which could be satisfied by either Ca2+ or Mn2+. Under the same conditions, La3+ was inhibitory. Triton X-100 caused loss of intravesicular Ca2+, yet this was accompanied by an increase in phosphate incorporation into the caseins. We conclude from these results that the binding site on casein kinase for ATP is within the Golgi membrane barrier and that they imply the presence of a transmembrane ATP-transport mechanism. Inhibition of casein phosphorylation by atractyloside and carboxyatractyloside lends support to this concept.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besch H. R., Jr, Jones L. R., Fleming J. W., Watanabe A. M. Parallel unmasking of latent adenylate cyclase and (Na+,K+)-ATPase activities in cardiac sarcolemmal vesicles. A new use of the channel-forming ionophore Alamethicin. J Biol Chem. 1977 Nov 25;252(22):7905–7908. [PubMed] [Google Scholar]
- Bingham E. W., Groves M. L. Properties of casein kinase from lactating bovine mammary gland. J Biol Chem. 1979 Jun 10;254(11):4510–4515. [PubMed] [Google Scholar]
- Bonnafous J. C., Dornand J., Mani J. C. Alamethicin or detergent permeabilization of the cell membrane as a tool for adenylate cyclase determination. Biochim Biophys Acta. 1982 Jun 8;720(3):235–241. doi: 10.1016/0167-4889(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Brew K. Secretion of alpha-lactalbumin into milk and its relevance to the organization and control of lactose synthetase. Nature. 1969 May 17;222(5194):671–672. doi: 10.1038/222671a0. [DOI] [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. The subcellular distribution of the A and B proteins of lactose synthetase in bovine and rat mammary tissue. J Biol Chem. 1966 Dec 10;241(23):5526–5532. [PubMed] [Google Scholar]
- Coffey R. G., Reithel F. J. The lactose synthetase particles of lactating bovine mammary gland. Preparation of particles with intact lactose synthetase. Biochem J. 1968 Sep;109(2):169–176. doi: 10.1042/bj1090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. A. Studies on the particulate lactose synthetase of mouse mammary gland and the role of -lactalbumin in the initiation of lactose synthesis. Biochem J. 1972 Jan;126(1):67–78. doi: 10.1042/bj1260067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. R., Maddock S. W., Besch H. R., Jr Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem. 1980 Oct 25;255(20):9971–9980. [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Effects of phlorrhizin and thiol reagents on the galactosyltransferase activity of Golgi membrane vesicles of lactating rat mammary gland. Biochem J. 1980 May 15;188(2):503–507. doi: 10.1042/bj1880503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., Wooding F. B., White A. Properties of galactosyltransferase-enriched vesicles of Golgi membranes from lactating-rat mammary gland. Eur J Biochem. 1980 Jan;103(2):377–385. doi: 10.1111/j.1432-1033.1980.tb04324.x. [DOI] [PubMed] [Google Scholar]
- Lad P. J., White A. A. Effect of alamethicin, gramicidin S and melittin upon the particulate guanylate cyclase from rat lung. Biochim Biophys Acta. 1979 Sep 12;570(1):198–209. doi: 10.1016/0005-2744(79)90214-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Jones L. R. Characterization of the intrinsic cAMP-dependent protein kinase activity and endogenous substrates in highly purified cardiac sarcolemmal vesicles. J Biol Chem. 1982 Sep 10;257(17):10052–10062. [PubMed] [Google Scholar]
- Quist E. E., Roufogalis B. D. Determination of the stoichiometry of the calcium pump in human erythrocytes using lanthanum as a selective inhibitor. FEBS Lett. 1975 Feb 1;50(2):135–139. doi: 10.1016/0014-5793(75)80473-x. [DOI] [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. An alternative membrane transport pathway for phosphate and adenine nucleotides in mitochondria and its possible function. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4788–4792. doi: 10.1073/pnas.75.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Tschabold M. The lanthanides Ho3+ and Pr3+ as inhibitors of calcium transport in human red cells. Experientia. 1971 Jan 15;27(1):59–61. doi: 10.1007/BF02137741. [DOI] [PubMed] [Google Scholar]
- Szymanski E. S., Farrell H. M., Jr Isolation and solubilization of casein kinase from Golgi apparatus of bovine mammary gland and phosphorylation of peptides. Biochim Biophys Acta. 1982 Apr 3;702(2):163–172. doi: 10.1016/0167-4838(82)90498-8. [DOI] [PubMed] [Google Scholar]
- Weiner M. L., Lee K. S. Active calcium ion uptake by inside-out and right side-out vesicles of red blood cell membranes. J Gen Physiol. 1972 Apr;59(4):462–475. doi: 10.1085/jgp.59.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. W., Clegg R. A. Casein kinase activity in rat mammary gland Golgi vesicles. Phosphorylation of endogenous caseins. Eur J Biochem. 1983 Dec 1;137(1-2):215–220. doi: 10.1111/j.1432-1033.1983.tb07817.x. [DOI] [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]


