Abstract
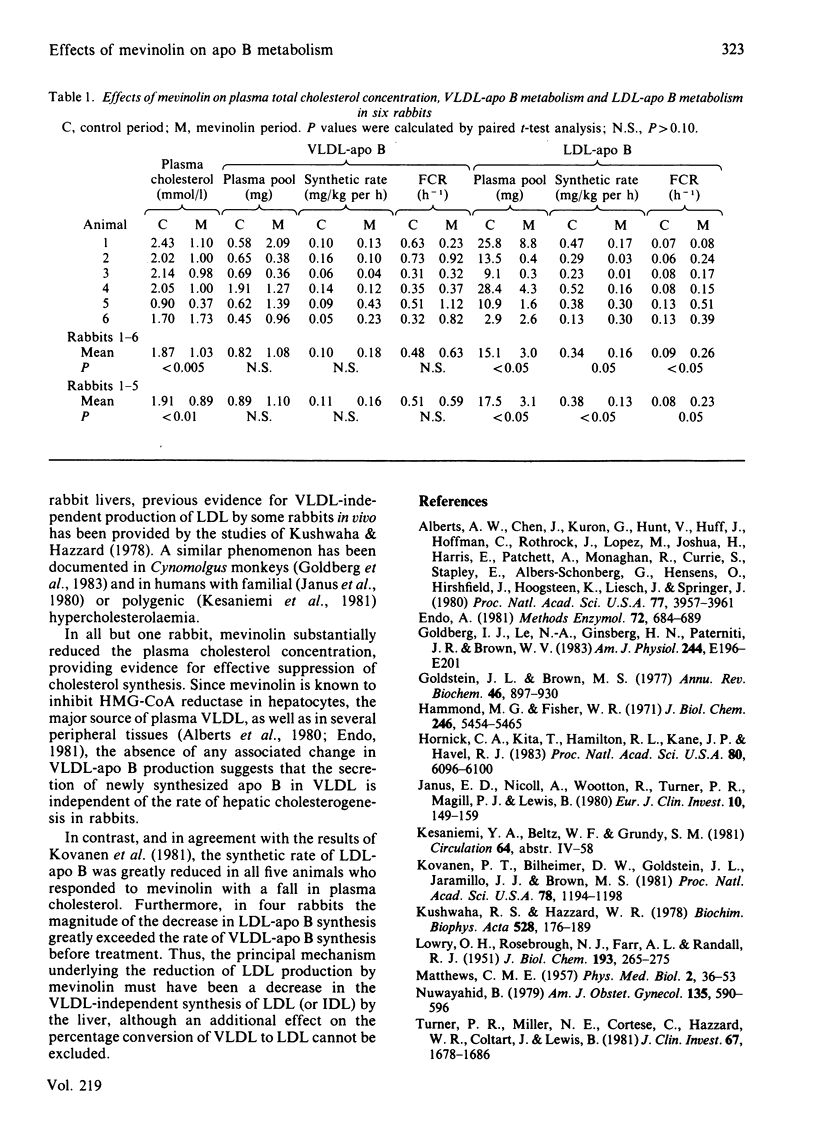
The kinetics of the apoprotein B (apo B) of very-low-density (VLDL; d less than 1.006) and low-density (LDL; d 1.019-1.063) lipoproteins were studied in six rabbits by using radioiodinated homologous lipoproteins, before and during oral administration of mevinolin (5 mg/kg per day), a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (EC 1.1.1.34), to explore the mechanism by which the drug reduces LDL synthesis. Before treatment LDL-apo B production greatly exceeded VLDL-apo B production in all animals, indicating that a large proportion of plasma LDL was derived from a VLDL-independent pathway. Five animals responded to mevinolin with a fall in plasma cholesterol (mean change - 53%; P less than 0.01). This was associated with a 66% decrease in LDL-apo B synthesis (P less than 0.05). In contrast, VLDL-apo B synthesis was unaffected by mevinolin. Furthermore, in all but one animal the decrement in LDL-apo B synthesis was greater than the rate of VLDL-apo B synthesis before treatment, demonstrating that mevinolin had reduced the VLDL-independent production of LDL.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors. Methods Enzymol. 1981;72:684–689. doi: 10.1016/s0076-6879(81)72058-5. [DOI] [PubMed] [Google Scholar]
- Goldberg I. J., Le N. A., Ginsberg H. N., Paterniti J. R., Jr, Brown W. V. Metabolism of apoprotein B in cynomolgus monkey: evidence for independent production of low-density lipoprotein apoprotein B. Am J Physiol. 1983 Feb;244(2):E196–E201. doi: 10.1152/ajpendo.1983.244.2.E196. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Hammond M. G., Fisher W. R. The characterization of a discrete series of low density lipoproteins in the disease, hyper-pre-beta-lipoproteinemia. Implications relating to the structure of plasma lipoproteins. J Biol Chem. 1971 Sep 10;246(17):5454–5465. [PubMed] [Google Scholar]
- Hornick C. A., Kita T., Hamilton R. L., Kane J. P., Havel R. J. Secretion of lipoproteins from the liver of normal and Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6096–6100. doi: 10.1073/pnas.80.19.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus E. D., Nicoll A., Wootton R., Turner P. R., Magill P. J., Lewis B. Quantitative studies of very low density lipoprotein: conversion to low density lipoprotein in normal controls and primary hyperlipidaemic states and the role of direct secretion of low density lipoprotein in heterozygous familial hypercholesterolaemia. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):149–159. doi: 10.1111/j.1365-2362.1980.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha R. S., Hazzard W. R. Catabolism of very low density lipoproteins in the rabbit. Effect of changing composition and pool size. Biochim Biophys Acta. 1978 Feb 27;528(2):176–189. doi: 10.1016/0005-2760(78)90192-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Nuwayhid B. Hemodynamic changes during pregnancy in the rabbit. Am J Obstet Gynecol. 1979 Nov 1;135(5):590–596. doi: 10.1016/s0002-9378(16)32982-9. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Miller N. E., Cortese C., Hazzard W., Coltart J., Lewis B. Splanchnic metabolism of plasma apolipoprotein B: studies of artery-hepatic vein differences of mass and radiolabel in fasted human subjects. J Clin Invest. 1981 Jun;67(6):1678–1686. doi: 10.1172/JCI110205. [DOI] [PMC free article] [PubMed] [Google Scholar]


