Abstract
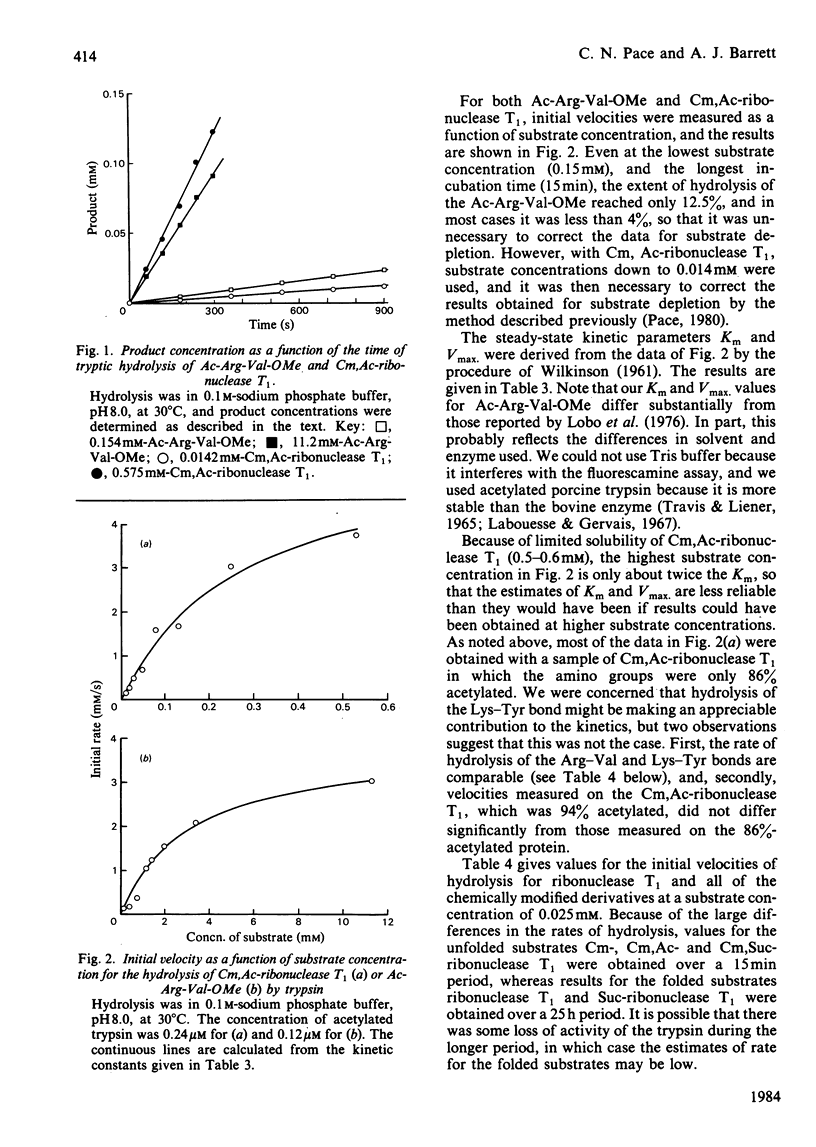
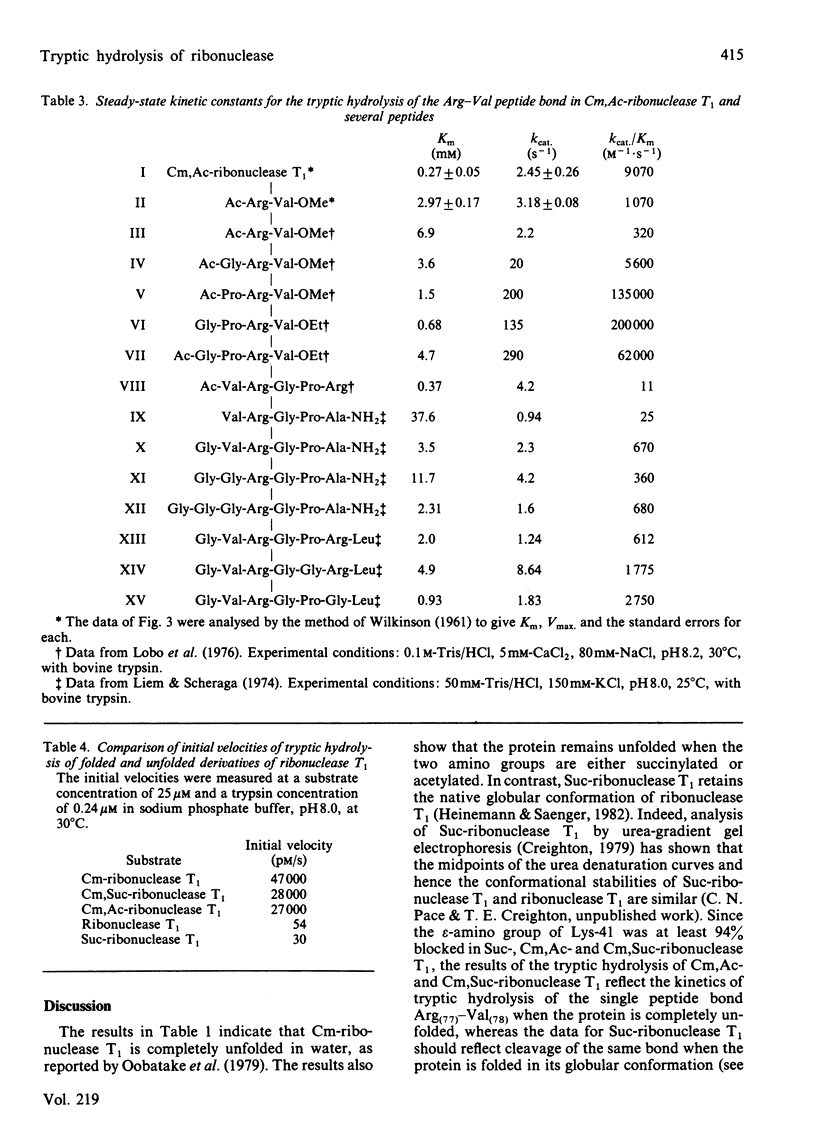
We have used ribonuclease T1 and its chemically modified derivatives as substrates, and trypsin as proteinase, to investigate the kinetics of proteolysis of a specific peptide bond in the folded and unfolded conformations of a protein. Steady-state kinetic studies showed that Km = 0.27 mM and Kcat. = 2.45 s-1 for the tryptic hydrolysis of the Arg(77)-Val(78) peptide bond in unfolded ribonuclease T1. This Km is somewhat lower than, and the kcat. value similar to, values found for the tryptic hydrolysis of comparable bonds in small peptides. Our data for the initial velocity of hydrolysis of the Arg(77)-Val(78) bond in a solution of the folded protein indicate that the bond is at least 1700 times less rapidly hydrolysed in the folded than in the unfolded conformation of ribonuclease T1, and do not exclude the possibility that the bond is completely resistant to hydrolysis in the folded protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Bigelow C. C. Estimation of the free energy of stabilization of ribonuclease A, lysozyme, alpha-lactalbumin, and myoglobin. J Biol Chem. 1982 Nov 10;257(21):12935–12938. [PubMed] [Google Scholar]
- Blundell T., Wood S. The conformation, flexibility, and dynamics of polypeptide hormones. Annu Rev Biochem. 1982;51:123–154. doi: 10.1146/annurev.bi.51.070182.001011. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Kinetic study of protein unfolding and refolding using urea gradient electrophoresis. J Mol Biol. 1980 Feb 15;137(1):61–80. doi: 10.1016/0022-2836(80)90157-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Bernardo S., Weigele M., Toome V., Manhart K., Leimgruber W., Böhlen P., Stein S., Udenfriend S. Studies on the reaction of fluorescamine with primary amines. Arch Biochem Biophys. 1974 Jul;163(1):390–399. doi: 10.1016/0003-9861(74)90490-1. [DOI] [PubMed] [Google Scholar]
- Fields R., Dixon H. B., Law G. R., Yui C. Purification of ribonuclease T 1 by diethylaminoethylcellulose chromatography. Biochem J. 1971 Feb;121(4):591–596. doi: 10.1042/bj1210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata C., Senma T., Saito D., Matsushima T., Sugimura T. A new fluorescent microassay method for pepsin using succinyl-albumin. Anal Biochem. 1978 Feb;84(2):479–485. doi: 10.1016/0003-2697(78)90066-0. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Saenger W. Specific protein-nucleic acid recognition in ribonuclease T1-2'-guanylic acid complex: an X-ray study. Nature. 1982 Sep 2;299(5878):27–31. doi: 10.1038/299027a0. [DOI] [PubMed] [Google Scholar]
- LINDERSTRØM-LANG K. Structure and enzymatic break-down of proteins. Cold Spring Harb Symp Quant Biol. 1950;14:117–126. doi: 10.1101/sqb.1950.014.01.016. [DOI] [PubMed] [Google Scholar]
- Labouesse J., Gervais M. Preparation of chemically defined epsilon N-acetylated trypsin. Eur J Biochem. 1967 Sep;2(2):215–223. doi: 10.1111/j.1432-1033.1967.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Scheraga H. A. Mechanism of action of thrombin on fibrinogen. IV. Further mapping of the active sites of thrombin and trypsin. Arch Biochem Biophys. 1974 Jan;160(1):333–339. doi: 10.1016/s0003-9861(74)80041-x. [DOI] [PubMed] [Google Scholar]
- Lobo A. P., Wos J. D., Yu S. M., Lawson W. B. Active site studies of human thrombin and bovine trypsin: peptide substrates. Arch Biochem Biophys. 1976 Nov;177(1):235–244. doi: 10.1016/0003-9861(76)90433-1. [DOI] [PubMed] [Google Scholar]
- Oobatake M., Takahashi S., Ooi T. Conformational stability of ribonuclease T1. II. Salt-induced renaturation. J Biochem. 1979 Jul;86(1):65–70. [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Pozsgay M., Szabó G., Bajusz S., Simonsson R., Gáspár R., Elödi P. Investigation of the substrate-binding site of trypsin by the aid of tripeptidyl-p-nitroanilide substrates. Eur J Biochem. 1981 Apr;115(3):497–502. doi: 10.1111/j.1432-1033.1981.tb06230.x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Sweet R. M., Wright H. T., Janin J., Chothia C. H., Blow D. M. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6-A resolution. Biochemistry. 1974 Sep 24;13(20):4212–4228. doi: 10.1021/bi00717a024. [DOI] [PubMed] [Google Scholar]
- TRAVIS J., LIENER I. E. THE CRYSTALLIZATION AND PARTIAL CHARACTERIZATION OF PORCINE TRYPSIN. J Biol Chem. 1965 May;240:1962–1966. [PubMed] [Google Scholar]
- Takahashi K., Inoue N. The structure and function of ribonuclease T1. XXII. Tryptic cleavages of the single lysyl and arginyl bonds in ribonuclease T1. J Biochem. 1977 Feb;81(2):415–421. doi: 10.1093/oxfordjournals.jbchem.a131473. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Specific modification of arginine residues in proteins with ninhydrin. J Biochem. 1976 Nov;80(5):1173–1176. doi: 10.1093/oxfordjournals.jbchem.a131373. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Uchida T., Egami F. Ribonuclease T1, Structure and function. Adv Biophys. 1970;1:53–98. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


