Abstract
Background
Red blood cell (RBC) transfusions play an important role in supportive care in children and neonates with cancer. However, in current clinical practice, evidence-based recommendations are lacking on when to administer prophylactic RBC transfusions. To address this gap, a clinical practice guideline (CPG) was developed to systematically review the available evidence and provide recommendations for clinicians.
Methods
A systematic literature review in three databases was conducted. The GRADE methodology was used to assess, extract, and summarize the evidence. A multidisciplinary panel of 21 professionals was assembled to ensure comprehensive expertise. If there was insufficient evidence in children with cancer, additional evidence was gathered in general pediatric or adult oncology guidelines, or the panel utilized shared expert opinion to develop a comprehensive CPG. Multiple in-person meetings were conducted to discuss evidence, complete evidence-to-decision frameworks, and formulate recommendations.
Results
Four studies including 203 children with all types of cancer, met the inclusion criteria. The expert panel assessed all evidence and translated it into recommendations. In total, 47 recommendations were formulated regarding RBC transfusions in children and neonates with cancer. For instance, specific thresholds for prophylactic RBC transfusions were recommended for children and neonates with cancer who have sepsis, are on ECMO, or are undergoing radiotherapy.
Conclusion
This clinical practice guideline presents evidence-based recommendations regarding RBC transfusions in children and neonates with cancer. By providing these recommendations, we aim to guide clinicians and contribute to improving outcomes for children and neonates with cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-024-08888-3.
Keywords: Pediatric oncology, Red blood cell transfusions, Clinical practice guideline
Introduction
Red blood cell (RBC) transfusions are important in the supportive care for children with cancer and those undergoing a hematopoietic stem cell transplantation (HSCT). These transfusions are often necessary due to anemia resulting from their underlying oncological disease or due to bone marrow depression during their anti-cancer treatment [1]. Blood transfusions can significantly improve the quality of life of children and neonates with cancer. However, while transfusions are generally well tolerated, they are associated with adverse short- and long-term effects (such as volume overload, transfusion reactions, and iron overload) [2, 3]. Thus, it is essential to strike a balance between unnecessary transfusions—and its adverse effects—and preventing complications caused by anemia.
Unfortunately, current clinical practice lacks evidence-based recommendations for administering RBC transfusions in children with cancer specifically. Given the frequency of these transfusions in these patients, it is crucial to critically review and assess the available evidence to develop accurate recommendations.
Therefore, our aim was to develop a clinical practice guideline (CPG) regarding RBC transfusions in children with all types of cancer in general and children with all types of cancer who are undergoing an HSCT. This CPG focuses on prophylactic RBC transfusions in children and neonates with cancer. We explicitly aimed to provide recommendations even in the absence of evidence, to establish good clinical practice and provide clinicians with a comprehensive guideline.
Methods
Guideline panel
A national, comprehensive multidisciplinary panel was assembled, comprising 22 professionals and a patient representative. The panel included pediatric hemato-oncologists, pediatricians, a radiotherapist, a surgeon, a patient representative, nurse specialists, a pediatric intensive care specialist, a laboratory specialist, guideline specialists, and several researchers (see Supplemental Materials S1). Members were invited on the basis of their experience and knowledge on the topic. The core group (DK, DS, RM, LK, WT, EL) provided all the preparatory documents including methodology, study details, and results. Between 2020 and 2022, multiple in-person meetings were held to rank outcomes, discuss the evidence, and formulate recommendations.
Guideline scope
This CPG includes recommendations regarding prophylactic RBC transfusions in children with cancer aged 0–18 years receiving anti-cancer treatment with curative intent. This guideline was not intended to provide recommendations for palliative care settings or for cases of ongoing blood loss (e.g., emergency care, ongoing blood loss in gastro-intestinal tract, epistaxis). The guideline focuses on prophylactic RBC transfusions; symptoms can however influence the threshold for transfusion and clinical decision-making accordingly.
Existing guidelines and clinical questions
Existing international guidelines on prophylactic RBC transfusions were searched (latest search February 2023: National Institute for Health and Care Excellence (NICE), Guidelines International Network (GIN), American Society of Clinical Oncology (ASCO), international Pediatric Oncology Group (iPOG), and Cancer Guideline Database) and evaluated for the applicability and completeness of these guidelines. Considering the absence of an applicable evidence-based guideline for children with cancer, clinical questions were formulated by the core group. An overview of the clinical questions is shown in the Supplemental Materials S2.
Search strategy and selection criteria
An extensive systematic literature search (shown in Supplemental Materials S3) was performed in collaboration with a medical librarian. We searched electronic databases PubMed, Embase, and Cochrane CENTRAL.
In- and exclusion criteria were predefined by the core group. Importantly, all children and neonates with all types of cancer aged 0 to 18 years were included. Studies were included if groups with different thresholds for RBC transfusions were compared. We only included controlled studies, applying a two-step approach by first including RCTs, but in case of insufficient or inconclusive evidence, we included other controlled studies. It was agreed upon that when there were not enough studies identified, we would extrapolate from evidence-based guidelines in other pediatric patient populations (e.g., benign hematology or cardiology) or guidelines in adult oncology patients (applicability depending on clinical question).
Primary evidence selection and quality assessment
Study identification was performed by title and abstract screening, followed by full text assessment, independently by two reviewers (DK, DS). Discrepancies were resolved by consensus.
Detailed information from each eligible study was extracted into evidence tables. The methodological quality of each single study was assessed and scored on risk of bias. For RCTs, the risk of bias tool v2 from the Cochrane handbook [4] was used. For non-RCT studies, we combined the risk of bias criteria for observational studies, as described in the Handbook of the International Guideline Harmonization Group [5], with specific aspects of the Cochrane RCT tool [4]. By combining these tools, we aimed to have the best possible tool to assess the risk of bias in our types of studies. These risk of bias assessment criteria for non-RCT studies and the risk of bias results are shown in the Supplemental Materials S4.
All the evidence was collected in summary of findings tables. Per outcome, the quality of the total body of evidence was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [6]. Data-extraction, risk of bias assessment, and GRADE assessment were independently performed by two reviewers (DK, DS). Discrepancies were resolved by consensus.
Additional evidence selection and quality assessment
In anticipation of a lack of studies in childhood cancer patients, we searched for additional evidence. Guidelines on RBC transfusions in children without cancer or adults with cancer were searched in PubMed, Joint United Kingdom Blood Transfusion Services Professional Advisory Committee (JPAC), NICE, GIN, ASCO, iPOG, and Dutch Federation of Medical Specialists (FMS). The quality of the guidelines was assessed according to the AGREE II [7] method. A guideline was eligible for inclusion if the AGREE II-score was 4 or higher (Supplemental Materials S5). The included single studies in those guidelines served as the evidence base for extrapolation. In addition, in case of lack of evidence, recommendations from high-quality guidelines are adopted.
Translating evidence into recommendations using the evidence-to-decision framework
The GRADE evidence-to-decision framework was used to translate evidence into recommendations [6]. Within this framework, for every clinical question, the benefits and harms, resource use, equity, acceptability, and feasibility were discussed, and recommendations were formulated by the guideline panel. If no studies were identified, we carefully considered expert consensus (expert opinion). All expert opinion recommendations can be interpreted on the level of “weak recommendations,” as the panel felt there were no expert opinion recommendations that should be labeled as “strong recommendation.” Final recommendations were unanimously supported by all panel members.
The GRADE terminology for evidence-based guidelines was used, such as “we suggest” or “we recommend” [6]. For the expert-based recommendations, the terminology from a recent paper published by the international Pediatric Oncology Guidelines in supportive care (iPOG) network [8] was used. The wording “we believe” was used to emphasize that these recommendations are based on expert opinion and group consensus. A color-coding system was used to improve understandability and to emphasize the strength of the recommendations [9].
Results
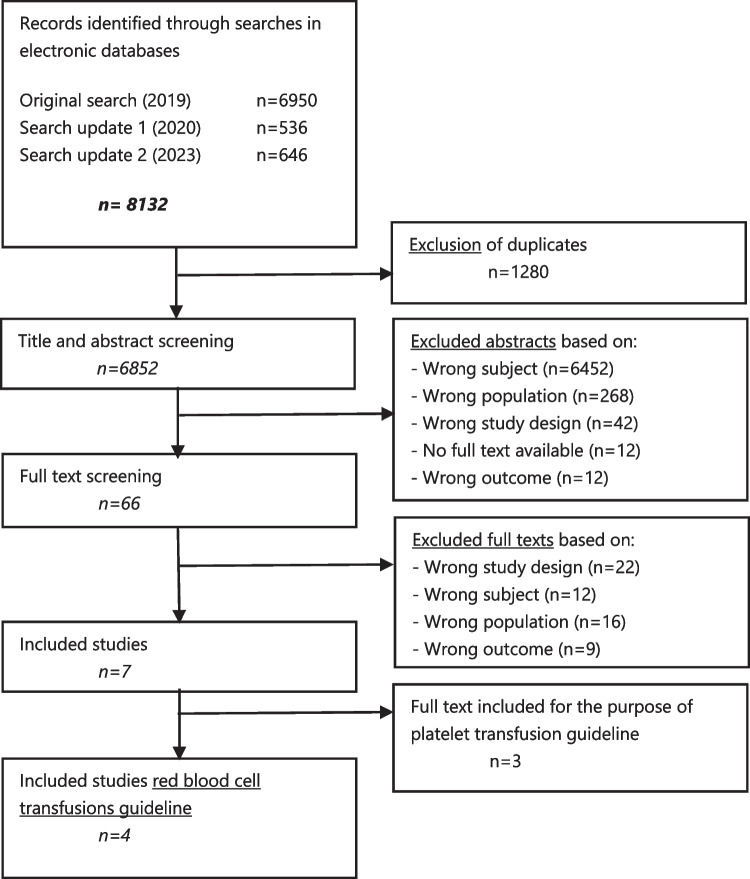
In total, 8132 unique citations were identified in initial literature search (September 2019) and two update searches (latest: February 2023), see flowchart 1.
Flowchart 1.
Flowchart of the inclusion and exclusion process (including the interim updates)
Four primary studies (3 RCTs, 1 pre-post trial) were included with a total number of 203 participants (see Fig. 1 in Supplemental Materials S6). All primary study characteristics and conclusions of evidence are shown in Supplemental Materials S6, including the inclusion and exclusion process. Moreover, seven (non-childhood cancer) guidelines were included with a total of 43 different single studies. An overview of the included studies, the conclusions of evidence, the evidence tables, and the GRADE assessments can be found in the Supplemental Materials S7. An overview of RBC transfusion recommendations for children and neonates with cancer is presented in Supplemental Materials S8. Within the overview of all recommendations, a color-coding system was used to improve understandability and to emphasize the strength of the recommendations. Below, all recommendations and their evidence-to-decision processes are discussed per subject. Given the number of recommendations and the extent of the supporting materials, only conclusions and important considerations of the guideline panel are shown. Full details, including the evidence to decision frameworks, are shown in the Supplemental Materials S9. The results section is divided into the different circumstances in which we recommend a prophylactic RBC transfusion. An overview of the recommendations for scientific research is included in Supplemental Materials S10.
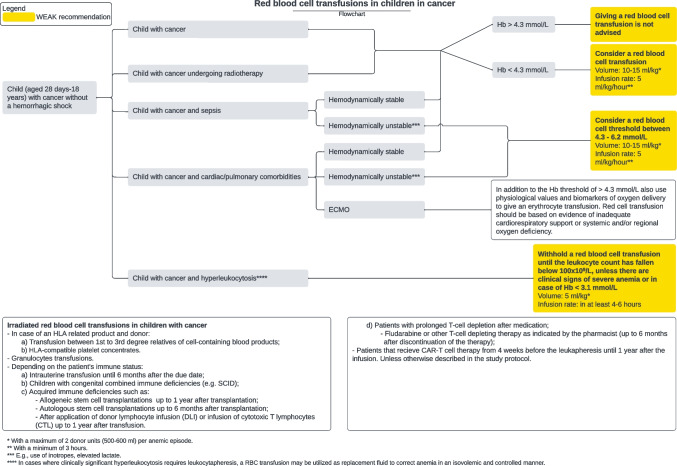
The recommendations on RBC transfusions for children and neonates with cancer are visualized below (Figs. 1 and 2). These flowcharts are also offered separately with measurements of Hb in g/dL.
Fig. 1.
Flowchart of RBC transfusion recommendations for children with cancer
Fig. 2.
Flowchart of RBC transfusion recommendations for neonates with cancer
Prophylactic red blood cell transfusion in general
Prophylactic red blood cell transfusion in children with cancer
Recommendation 1.1.1.
We suggest a hemoglobin (Hb) threshold of 4.3 mmol/L for RBC transfusion in children with cancer. (weak recommendation, very low quality evidence).
Recommendation 1.1.2.
We suggest against an Hb threshold of 3.7 mmol/L for RBC transfusion in children with cancer. (weak recommendation, very low quality evidence).
Recommendation 1.1.3.
We recommend against an Hb threshold of 3.1 mmol/L or lower for RBC transfusion in children with cancer. (strong recommendation, very low quality evidence).
Recommendation 1.1.4.
We suggest against an Hb threshold greater than 4.3 mmol/L for RBC transfusion in children with cancer. (weak recommendation, very low quality evidence).
Evidence to decision
The comparison of an Hb threshold of 4.3 mmol/L to an Hb threshold greater than 4.3 mmol/L involved two pediatric oncology studies, one pediatric non-cancer study, and five adult non-cancer studies. Apart from significantly lower costs, there was no significant increased risk for mortality, morbidity, and transfusion-related complications with a threshold Hb of 4.3 mmol/L in comparison to an Hb threshold greater than 4.3 mmol/L in children with cancer (VERY LOW quality of evidence) [1, 10]. From the guidelines that included single studies with children in general and adults, one adult study reported significantly higher mortality in the group with an Hb < 4.3 mmol/L in comparison to an Hb > 4.3 mmol/L in group [11]. Another adult study reported significantly lower mortality in the group with an Hb < 4.3 mmol/L in comparison to an Hb > 4.3 mmol/L in group [12], while six other pediatric studies with cancer and adult studies reported no significant difference in mortality [1, 10, 12–15]. Based on the available evidence, the panel concluded that there is likely no increased mortality risk. Additionally, two studies demonstrated fewer infections with an Hb threshold of 4.3 mmol/L compared to an Hb threshold greater than 4.3 mmol/L [12, 16]. Furthermore, there was no significant increase in quality of life with a higher Hb threshold than 4.3 mmol/L [12]. Considering these findings, the guideline panel determined that the benefits of maintaining an Hb threshold of 4.3 mmol/L compared to an Hb threshold greater than 4.3 mmol/L are likely substantial. Therefore, we suggest an Hb threshold of 4.3 mmol/L in children with cancer. Moreover, no other study reported significant increase in benefits or harms from a higher Hb threshold, such as 5.0 mmol/L [1, 12, 14, 16–19]. Also, the guideline panel considered the potential risks of iron overload and increased costs associated with a higher Hb threshold, and therefore, we suggest against adopting an Hb threshold greater than 4.3 mmol/L.
Regarding the comparison of an Hb threshold of 3.7 mmol/L to an Hb threshold greater than 3.7 mmol/L, no pediatric oncology studies were found. However, there were two adult non-cancer studies identified from the included guidelines. Pooled results indicated a significantly increased mortality risk in adult patients with an Hb threshold of 3.7 mmol/L in comparison to an Hb threshold greater than 3.7 mmol/L [11, 13]. Similar to the previous comparison, no studies reported any potential benefit from an Hb threshold of 3.7 mmol/L. Therefore, we suggest against an Hb threshold of 3.7 mmol/L.
Regarding the comparison of an Hb threshold of 3.1 mmol/L to an Hb threshold greater than 3.1 mmol/L, no pediatric oncology studies were found. However, there were three adult non-cancer studies and one pediatric non-cancer study identified from the included guidelines. These studies consistently reported significantly higher mortality rates in hospitalized adults and children with an Hb of 3.1 mmol/L [11, 13, 20, 21]. Despite the low level of evidence, which is mainly derived from adult studies, the guideline panel strongly advised against offering this option due to the higher mortality rates.
Prophylactic red blood cell transfusion in neonates with cancer
Recommendation 1.2.1.
We suggest an Hb threshold of 6.5 mmol/L for RBC transfusion in neonates with cancer when they are less than 1 week old.
(weak recommendation, very low quality evidence).
Recommendation 1.2.2.
We suggest an Hb threshold of 5.5 mmol/L for RBC transfusion in neonates with cancer when they are between 1 and 3 weeks old.
(weak recommendation, very low quality evidence).
Recommendation 1.2.3.
We suggest an Hb threshold of 4.5 mmol/L for RBC transfusion in neonates with cancer when they are between 3 and 4 weeks old.
(weak recommendation, very low quality evidence).
Evidence to decision
The incidence of cancer in neonates is exceedingly low. Despite this, it is crucial to provide recommendations for this specific patient group. Unfortunately, no pediatric oncology studies were identified to inform the guideline panel’s decision. However, the Dutch Association of Medical Specialists (FMS) [22] developed a high-quality guideline addressing this matter, receiving an AGREE II-score of 6 out of 7. They provided recommendations primarily based on studies conducted in very low birth-weight infants (birth weight of 1500 g or less). Although evidence specific to full-term and late-premature neonates (gestational age ≥ 32 weeks) is lacking, the FMS has adopted these thresholds for neonates in general. Considering the lack of evidence, the guideline panel decided to adopt the recommendations regarding neonates with cancer from the guideline of the FMS (2019).
Prophylactic red blood cell transfusion—sepsis
Prophylactic red blood cell transfusion in children with cancer during sepsis
Recommendation 2.1.1.
We suggest an Hb threshold of 4.3 mmol/L for RBC transfusion in children with cancer during sepsis who are hemodynamically stable.
(weak recommendation, very low quality evidence).
Recommendation 2.1.2.
We believe that for hemodynamically unstable children with cancer during sepsis and evidence of oxygen deficiency (e.g., use of inotropes, elevated lactate), an Hb threshold that ranges between 4.3 and 6.2 mmol/L should be considered.
(expert opinion).
Evidence to decision
Regarding children with cancer during sepsis who are hemodynamically stable, one pediatric non-cancer study and one adult non-cancer study were identified. Based on this limited evidence, there is no suggestions that there is an increased risk for mortality or morbidity with an Hb threshold of 4.3 mmol/L in comparison to an Hb threshold greater than 4.3 mmol/L in children and adults with sepsis who are clinically stable [17, 23]. Furthermore, no studies reported any significant potential benefit from an Hb threshold greater than 4.3 mmol/L [17]. Therefore, we suggest an Hb threshold of 4.3 mmol/L in children with cancer during sepsis who are hemodynamically stable. However, in hemodynamically unstable children with cancer during sepsis and evidence of oxygen deficiency (e.g., use of inotropes, elevated lactate), it is suggested to consider an Hb threshold ranging between 4.3 mmol/L and 6.2 mmol/L as part of a comprehensive approach to improve oxygen delivery for children with unstable non hemorrhagic shock and evidence of oxygen debt (WEAK recommendation) [24].
Prophylactic red blood cell transfusion in neonates with cancer during sepsis
Recommendation 2.2.1.
We suggest an Hb threshold of 6.5 mmol/L for RBC transfusion in neonates with cancer during sepsis when they are less than 1 week old.
(weak recommendation, very low quality evidence).
Recommendation 2.2.2.
We suggest an Hb threshold of 5.5 mmol/L for RBC transfusion in neonates with cancer during sepsis when they are between 1 and 3 weeks old.
(weak recommendation, very low quality evidence).
Recommendation 2.2.3.
We suggest an Hb threshold of 4.5 mmol/L for RBC transfusion in neonates with cancer during sepsis when they are between 3 and 4 weeks old.
(weak recommendation, very low quality evidence).
Evidence to decision
There were no studies found on neonates with cancer during sepsis. There was no suggestion for an increased risk for mortality and morbidity in hemodynamically stable children and adults with sepsis with an Hb threshold of 4.3 mmol/L in comparison to an Hb threshold greater than 4.3 mmol/L (“Prophylactic red blood cell transfusion in children with cancer during sepsis” section) [17, 23, 24]. Therefore, we concluded that children with sepsis do not derive additional benefits from a higher Hb threshold compared to children without sepsis. Based on these findings, and the absence of direct evidence in neonates with sepsis, the guideline panel determined that the recommendations for neonates with cancer can be applied to neonates with cancer during sepsis as well (“Prophylactic red blood cell transfusion in neonates with cancer” section).
Prophylactic red blood cell transfusion—radiotherapy
Prophylactic red blood cell transfusion in children who undergo radiotherapy
Recommendation 3.1.1.
We believe an Hb threshold of 4.3 mmol/L for RBC transfusion should be maintained in children with cancer who undergo radiotherapy.
(expert opinion).
Evidence to decision
No studies specifically addressing children with cancer undergoing radiotherapy were identified. Several other studies including adults with cancer concluded that there was no improvement in outcomes with an Hb threshold greater than 4.3 mmol/L [25–28]. Therefore, we suggest an Hb threshold of 4.3 mmol/L for RBC transfusion in children with cancer who undergo radiotherapy.
Prophylactic red blood cell transfusion in neonates who undergo radiotherapy
Recommendation 3.2.1.
We believe an Hb threshold of 6.5 mmol/L for RBC transfusion should be maintained in neonates with cancer who undergo radiotherapy when they are less than 1 week old.
(expert opinion).
Recommendation 3.2.2.
We believe an Hb threshold for RBC transfusion of 5.5 mmol/L should be maintained in neonates with cancer who undergo radiotherapy when they are between 1 and 3 weeks old.
(expert opinion).
Recommendation 3.2.3.
We believe an Hb threshold for RBC transfusion of 4.5 mmol/L should be maintained in neonates with cancer who undergo radiotherapy when they are between 3 and 4 weeks old.
(expert opinion).
Evidence to decision
No specific studies in neonates with cancer were identified. For the considerations of the recommendations we refer to “Prophylactic red blood cell transfusion in children who undergo radiotherapy” section.
Prophylactic red blood cell transfusion—cardiac and pulmonary comorbidities
Prophylactic red blood cell transfusion in children with cancer with cardiac and/or pulmonary comorbidities
Recommendation 4.1.1.
We suggest an Hb threshold of 4.3 mmol/L for RBC transfusion in children with cancer and cardiac and/or pulmonary comorbidities.
(weak recommendation, very low quality evidence).
Recommendation 4.1.2.
We believe that in case of a hemodynamically unstable child with cancer and pulmonary and/or cardiac comorbidities (e.g., use of inotropes, elevated lactate), a higher Hb threshold can be considered.
(weak recommendation, very low quality evidence).
Recommendation 4.1.3.
For children on ECMO:
In critically ill children on ECMO, there is insufficient evidence to recommend a specific RBC transfusion decision-making strategy using physiologic-based metrics and biomarkers.
In critically ill children on ECMO, we believe in using physiologic metrics and biomarkers of oxygen delivery in addition to Hb concentration to guide RBC transfusion. Administration of a RBC transfusion should be based on evidence of inadequate cardiorespiratory support or decreased systemic and/or regional oxygen delivery.
(expert opinion).
Evidence to decision
No pediatric oncology studies were identified. Two pediatric non-cancer studies and one adult non-cancer study were identified. The evidence gathered from these studies indicated that there is no increased risk for mortality, morbidity, and hospital admission with an Hb threshold of 4.3 mmol/L compared to an Hb threshold greater than 4.3 mmol/L in children and adults with cardiac and pulmonary comorbidities [11, 17, 29]. Studies comparing higher restrictive Hb thresholds (such as 5.0 mmol/L or 5.6 mmol/L) also did not report significant better outcomes regarding mortality, morbidity, quality of life, and admission to hospital [19, 30, 31]. Therefore, the guideline panel decided to suggest an Hb threshold of 4.3 mmol/L in children with cancer and cardiac and pulmonary comorbidities. For hemodynamically unstable children with cancer and pulmonary and/or cardiac comorbidities, such as those requiring inotropes or exhibiting elevated lactate levels, an Hb threshold ranging between 4.3 and 6.2 mmol/L is considered. Regarding children on extracorporeal membrane oxygenation (ECMO), the guideline panel decided to adopt the recommendations stated above from the Valentine (2018) guideline [32], AGREE-II score 5 out of 7.
Prophylactic red blood cell transfusion in neonates with cancer with cardiac and/or pulmonary comorbidities
Recommendation 4.2.1.
We suggest an Hb threshold of 7.5 mmol/L for RBC transfusion in neonates with cancer and cardiac and pulmonary comorbidities when they are less than 1 week old.
(expert opinion).
Recommendation 4.2.2.
We suggest an Hb threshold of 6.5 mmol/L for RBC transfusion in neonates with cancer and cardiac and pulmonary comorbidities when they are between 2 and 3 weeks old.
(expert opinion).
Recommendation 4.2.3.
We suggest an Hb threshold of 5.5 mmol/L for RBC transfusion in neonates with cancer and cardiac and pulmonary comorbidities when they are between 3 and 4 weeks old.
(expert opinion).
Evidence to decision
No pediatric oncology studies addressing this clinical question were found. However, the Dutch Association of Medical Specialists (FMS) [22] developed recommendations primarily based on studies conducted in very low birth-weight infants (birth weight of 1500 g or less) who required respiratory support. Although evidence specific to full-term and late-premature neonates (gestational age ≥ 32 weeks) is lacking, the FMS has adopted these thresholds for neonates requiring respiratory support. Taking this into account, the guideline panel decided to adopt the recommendations regarding neonates with cancer and pulmonary and/or cardiac comorbidities from the guideline of the FMS (2019).
Prophylactic red blood cell transfusion—hyperleukocytosis
Prophylactic red blood cell transfusion in children with cancer during hyperleukocytosis
Recommendation 5.1.1.
In children with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint until the number of leukocytes has fallen below 100 × 109 /L or in the presence of clinical symptoms of hyperleukocytosis.
Recommendation 5.1.2.
In children with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint, unless there are severe clinical signs of anemia or in case of an Hb below 3.1 mmol/L.
Recommendation 5.1.3.
If needed, transfuse with a maximum of 5 ml/kg/4–6 h.
(expert opinion).
Evidence to decision
No specific studies addressing this topic were identified. However, a study focusing on the management of hyperleukocytosis in children and adults with cancer provided relevant information. According to this study, the use of RBC transfusions in such cases should generally be avoided due to the potential increase in blood viscosity and the associated risk of leukostasis development or exacerbation, unless the patient exhibits symptoms of anemia [33]. The guideline panel decided to take this into consideration in order to make a recommendation based on expert opinion. However, in cases where clinically significant hyperleukocytosis requires leukocytapheresis, a RBC transfusion may be utilized as replacement fluid to correct anemia in an isovolemic and controlled manner [34].
Prophylactic red blood cell transfusion in children and neonates with cancer during hyperleukocytosis
Recommendation 5.2.1.
In neonates with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint until the number of leukocytes has fallen below 100 × 109 /L or in the presence of clinical symptoms of hyperleukocytosis.
Recommendation 5.2.2.
In neonates with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint unless there are severe clinical signs of anemia or in case of an Hb below 5.5 mmol/L in neonates with cancer when they are less than 1 week old.
Recommendation 5.2.3.
In neonates with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint unless there are severe clinical signs of anemia or in case of an Hb below 4.5 mmol/L for RBC transfusion in neonates with cancer when they are between 1 and 3 weeks old.
Recommendation 5.2.4.
In neonates with cancer and hyperleukocytosis, we believe that a RBC transfusion should be given with restraint unless there are severe clinical signs of anemia or in case of an Hb below 3.5 mmol/L for RBC transfusion in neonates with cancer when they are between 3 and 4 weeks old.
Recommendation 5.2.5.
If needed, transfuse with a maximum of 5 ml/kg/4–6 h.
(expert opinion).
Evidence to decision
No specific studies addressing this topic were identified. For the considerations of the recommendations we refer to “Prophylactic red blood cell transfusion in children with cancer during hyperleukocytosis” section. The RBC thresholds were based on expert opinions.
Irradiated red blood cell transfusions
Irradiated red blood cell transfusions in children and neonates with cancer
Recommendation 6.1.1.
We believe that irradiated blood products should be used in case of an HLA-related product and donor:
-
Transfusion between 1st and 3rd degree relatives of cell-containing blood products
(expert opinion).
Recommendation 6.1.2.
We believe that irradiated blood products should be used in case of granulocyte transfusions.
(expert opinion).
Recommendation 6.1.3.
We believe that irradiated blood products should be used depending on the patient’s immune status:
During intrauterine transfusions until 6 months after the due date;
Children with congenital combined immune deficiencies (e.g., SCID); and
- Acquired immune deficiencies such as:
- Allogeneic stem cell transplantations up to 1 year after transplantation;
- Autologous stem cell transplantations up to 6 months after transplantation; and
- After application of donor lymphocyte infusion (DLI) or infusion of cytotoxic T lymphocytes (CTL) up to 1 year after transfusion.
(expert opinion).
Recommendation 6.1.4.
We believe that irradiated blood products should be used in case of patients with prolonged T cell depletion after medication:
Fludarabine or other T cell depleting therapy as indicated by the pharmacist (up to 6 months after discontinuation of the therapy)
Recommendation 6.1.5.
We believe that irradiated blood products should be used in case of patients that receive CAR-T cell therapy from 4 weeks before the leukapheresis until 1 year after the infusion. Unless otherwise described in the study protocol.
(expert opinion).
Evidence to decision
There were no pediatric oncology studies identified. However, the Dutch Association of Medical Specialists (FMS) (21) developed a high-quality guideline addressing this matter. The guideline drew its recommendations from a study of Kopolovic (2015) [35] and a survey among hemovigilance organizations worldwide. Considering the lack of evidence, the guideline panel decided to adopt the recommendations regarding irradiated blood products from the guideline of the FMS (2019) [22]. The guideline panel added the indication for the use of CAR-T cells, based on the recommendations in the current study protocol (the pharmaceutical company that creates the CAR-T cells prescribed this period of irradiated blood products in a research context).
Low- or high-volume red blood cell transfusions
Low- or high-volume red blood cell transfusions in children with cancer
Recommendation 7.1.1.
We suggest a transfusion volume of 10–15 ml/kg in children with cancer.
(weak recommendation, very low quality evidence).
Recommendation 7.1.2.
We suggest against a transfusion volume of 20 ml/kg or higher in children with cancer.
(weak recommendation, very low quality evidence).
Recommendation 7.1.3.
We suggest a transfusion volume with a maximum of 2 donor units (between 500 and 600 ml) per anemic episode.
(expert opinion).
Evidence to decision
No pediatric oncology studies were identified. In comparing a RBC transfusion volume of 10 ml/kg to a volume higher than 10 ml/kg, no studies including children were identified. However, one study involving neonates without cancer was identified. The limited evidence available suggests that there is no significant increase in morbidity associated with a transfusion volume of 10 ml/kg compared to a volume higher than 10 ml/kg [36]. Regarding the comparison of a volume of 15 ml/kg to a volume higher than 15 ml/kg, again, no studies including children were identified. However, two studies with neonates were identified. The available evidence suggests that there is no significant increase in mortality or morbidity associated with a transfusion volume of 15 ml/kg compared to a volume higher than 15 ml/kg [37, 38]. One study involving children without cancer compared a RBC transfusion volume of 20 ml/kg to a volume higher than 20 ml/kg [39]. The limited evidence available suggested that there is no significant difference in terms of mortality or morbidity when comparing a transfusion volume of 20 ml/kg to a volume higher than 20 ml/kg [39]. Additionally, the expert panel considered that a lower transfusion volume leads to reduced risk of volume overload and deemed this option as probably acceptable for all stakeholders. Therefore, we suggest in favor of a transfusion volume of 10–15 ml/kg, and suggest against the use of a volume of 20 ml/kg. The expert panel advises transfusing with a maximum of 2 donor units per anemic episode, which corresponds to a volume between 500 and 600 ml, based on shared expert opinion.
Low or high-volume red blood cell transfusions in neonates with cancer
Recommendation 7.2.1.
We suggest a transfusion volume of 10–15 ml/kg in neonates with cancer.
(weak recommendation, very low quality evidence).
Recommendation 7.2.2.
We suggest against a transfusion volume of 20 ml/kg or higher in neonates with cancer.
(weak recommendation, very low quality evidence).
Evidence to decision
For the considerations of the recommendations, we refer to “Low or high-volume RBC transfusion in children with cancer” section.
Infusion rates of red blood cell transfusions
Infusion rates of red blood cell transfusions in children with cancer
Recommendation 8.1.1.
We believe that the infusion rate of a RBC transfusion should be 5 ml/kg/h in children with cancer, with a minimum of 3 h.
(expert opinion).
Evidence to decision
There are no studies regarding infusion rates. However, JPAC [40] has provided a recommendation for an infusion rate of 5 ml/kg/h in children, based on consensus, AGREE II-score of 4 out of 7. The guideline panel decided to adopt this recommendation, but added to the advice that a transfusion should take at least 3 h, based on expert-opinions.
Infusion rates of red blood cell transfusions in neonates with cancer
Recommendation 8.2.1.
We believe that the infusion rate of a RBC transfusion should be 5 ml/kg/h in neonates with cancer.
(expert opinion).
Evidence to decision
No specific studies were identified regarding infusion rates. However, the Dutch Association of Medical Specialists [22] has provided a recommendation for an infusion rate of 5 ml/kg/h in neonates, based on consensus. The guideline panel decided to adopt this recommendation.
Discussion
This clinical practice guideline comprises recommendations, in line with the GRADE methodology [6], regarding prophylactic RBC transfusions in children and neonates with cancer and has the potential to provide valuable guidance for clinicians in daily practice and contribute to improving quality of life for children and neonates with cancer worldwide.
The most notable limitation of this CPG is the substantial lack of evidence regarding appropriate thresholds for prophylactic RBC transfusions in children and neonates with cancer. To address this limitation, we conducted comprehensive and extensive literature searches, including exploration of RBC transfusion guidelines for children without cancer and (young) adults with cancer. Unfortunately, the yield of relevant evidence was still remarkably low. However, the consensus among the guideline panel was unanimous in their determination to come up with recommendations even in the absence of adequate evidence from the literature. This was deemed essential, as healthcare providers in daily practice rely on practice guidelines to guide decision-making regarding transfusions in their patients. Consequently, the guideline panel incorporated recommendations from existing high-quality guidelines regarding RBC transfusions for adults with cancer and children in general in order to formulate recommendations based on the best available evidence. When such guidelines were unavailable, recommendations were constructed through expert consensus. We firmly believe that the incorporation of expert opinions serves as a valuable asset in enhancing clinical practice and should find more frequent implementation in the development of guidelines when evidence gaps exist.1 Nevertheless, with prophylactic RBC transfusions being administered so frequently in children and neonates with cancer and the potential serious consequences of anemia, it is abundantly clear that further research in this field is imperative.
A second limitation of our guideline is the composition of the guideline panel, which consisted of experts from a national level. While this panel provided valuable insights and expertise, it is important to consider the applicability of this guideline to local contexts. However, we have provided extensive supplemental materials and evidence-to-decision frameworks that allow clinicians to assess the relevance and applicability of the guidelines to their specific settings. This approach empowers clinicians in other countries to make informed decisions based on the available evidence and adapt the recommendations as needed for their local context.
Implementation of this evidence-based guideline holds promise for enhancing the quality of life in children and neonates with cancer. With these evidence- and expert-based recommendations, we have endeavored to provide comprehensive and practical guidance. To ensure transparency, we have meticulously documented all the considerations in the evidence-to-decision frameworks. The inclusion of evidence-to-decision frameworks in this guideline provides clinicians with a valuable tool to assess the individual benefits and harms associated with different treatment options for each child and are making the decision-making process transparent. We sincerely hope that this guideline serves as a valuable tool in balancing the benefits and risks, promoting cautiousness and restrictiveness where appropriate.
In conclusion, through the effective implementation of the recommendations outlined in this CPG, the guideline panel aims to improve care provided to children and neonates with cancer and contribute to enhancing their quality of life. These recommendations hold significant importance in current clinical practice, and we hope that the lack of evidence in this area will serve as a stimulus for further research efforts. We are currently developing indicators to monitor the impact of this guideline and to facilitate continuous evaluation and improvement of care in this field.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 9 KB)
Supplementary file2 (DOCX 7 KB)
Supplementary file3 (DOCX 10 KB)
Supplementary file4 (DOCX 103 KB)
Supplementary file5 (DOCX 19.2 KB)
Supplementary file6 (DOCX 755 KB)
Supplementary file7 (DOCX 81.6 KB)
Supplementary file8 (DOCX 19 KB)
Supplementary file9 (DOCX 753 KB)
Supplementary file10 (DOCX 7 KB)
Supplementary file11 (DOCX 455 KB)
Acknowledgements
We thank all the members of the guideline panel for their dedicated efforts and invaluable contributions to the development of this guideline over the past years. Your expertise and commitment have been instrumental in shaping a guideline that will make a positive impact in our field. Thank you for your unwavering dedication towards advancing best practices and improving care for children with cancer.
On behalf of the prophylactic red blood cell transfusion guideline panel: Dorine Bresters1, Janneke H.P. Evers1, Sjef P.J. van Gestel3, Melanie M. Hagleitner1, Katja M.J. Heitink-Pollé1, Elise J. Huisman4,5, Geert O.R. Janssens1,6, Philip H.M. Kuijper7, Maarten O. Mensink1, Joppe Nijman3, Jeroen G. Noordzij8, Ida Ophorst1, Willemijn Plieger9, Judith Spijkerman1, Alida F.W. van der Steeg1, Marianne D. van de Wetering1
1Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands
3Department of Pediatric Intensive Care, Wilhelmina Children’s Hospital, Utrecht, The Netherlands
4Department of Pediatric Hematology, Erasmus Medical Center-Sophia Children’s Hospital, Rotterdam, The Netherlands
5Unit Transfusion Medicine, Sanquin, Amsterdam, The Netherlands
6Department of Radiotherapy, Wilhelmina Children’s Hospital, Utrecht, The Netherlands
7Hematology Laboratory, Máxima Medical Center, Veldhoven, The Netherlands
8Department of Pediatrics, Reinier de Graaf Gasthuis, Delft, The Netherlands
9Representative of VKKN, Vereniging Kinderkanker Nederland, Patients and Parents’ Representative Organization, The Netherlands
Author contribution
Demi M. Kruimer (part of the core guideline panel): performed the search, reviewed the evidence, presented it to the whole guideline panel, and wrote the main manuscript.
Debbie C Stavleu (part of the core guideline panel): performed the search, reviewed the evidence, and gave feedback to the manuscript.
Renée L. Mulder (part of the core guideline panel): reviewed the evidence and gave feedback to the manuscript.
Leontien C.M. Kremer (part of the core guideline panel): reviewed the evidence and gave feedback to the manuscript.
Wim J.E. Tissing (part of the core guideline panel): reviewed the evidence and gave feedback to the manuscript.
Erik A.H. Loeffen (part of the core guideline panel): performed the search, reviewed the evidence, and revised and gave feedback to the manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Important note: The content of this guideline includes expert opinions and recommendations related to blood transfusions. It is crucial to understand that these expert opinions are guiding principles and are intended to serve as a reference for medical decision-making. They do not constitute legally binding regulations and are not meant to compel medical professionals to deviate from their professional judgment in any way. Medical professionals retain the right to, in accordance with their professional assessment, deviate from the recommendations outlined herein. The use of this guideline in legal proceedings should be approached with caution, as it serves as a guide for medical practice and does not impose absolute standards. Always consult your own legal counsel or medical professionals for specific legal or medical issues.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
The views expressed in the submitted article are those of the authors and guideline panel and might not reflect the official position of their institution. Funding from the SKMS was received for the development of this guideline, but the SKMS had no influence on the process, the outcomes, or the decision to submit this article for publication.
Contributor Information
Erik A. H. Loeffen, Email: eah.loeffen@umcg.nl
On behalf of the prophylactic red blood cell transfusion guideline panel:
Dorine Bresters, Janneke H. P. Evers, Sjef P. J. van Gestel, Melanie M. Hagleitner, Katja M. J. Heitink-Pollé, Elise J. Huisman, Geert O. R. Janssens, Philip H. M. Kuijper, Maarten O. Mensink, Joppe Nijman, Jeroen G. Noordzij, Ida Ophorst, Willemijn Plieger, Judith Spijkerman, Alida F. W. van der Steeg, and Marianne D. van de Wetering
References
- 1.Lightdale JR, Randolph AG, Tran CM, Jiang H, Colon A, Houlahan K, Lehmann LE (2012) Impact of a conservative red blood cell transfusion strategy in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 18(5):813–817. 10.1016/j.bbmt.2011.10.043 [DOI] [PubMed] [Google Scholar]
- 2.Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, Jacobs B, Markovitz B, Goldstein B, Hanson JH, Li HA, Randolph AG (2008) Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 178(1):26–33. 10.1164/rccm.200711-1637oc [DOI] [PubMed] [Google Scholar]
- 3.Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, Politi P, Durazzi SMT, Muretto P, Albertini F (1990) Bone marrow transplantation in patients with thalassemia. N Engl J Med 322(7):417–421. 10.1056/nejm199002153220701 [DOI] [PubMed] [Google Scholar]
- 4.Cochrane handbook for systematic reviews of interventions (2011) Cochrane handbook for systematic reviews of interventions. https://handbook-5-1.cochrane.org/. Accessed 14 Jul 2023.
- 5.Mulder RL, Brown MC, Skinner R, van Dalen EC, Hudson MM, Kremer LCM (2021) Handbook for guideline development; collaboration between International Guideline Harmonization Group, PanCare Guideline Group and Cochrane Childhood Cancer
- 6.Schünemann H, Brożek J, Guyatt G, Oxman A (2013) GRADE handbook. GRADE handbook. https://netherlands.cochrane.org/sites/netherlands.cochrane.org/files/public/uploads/6_agree_ii_dutch.pdf. Accessed 14 Jul 2023.
- 7.AGREE Next Steps Consortium. Appraisal of Guidelines for Research & Evaluation (AGREE) II Instrument. www.agreetrust.org. Accessed 14 Jul 2023.
- 8.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R, Barrington K, Roberts RS (2006) The premature infants in need of transfusion (pint) study: a randomized, controlled trial of a restrictive (LOW) versus liberal (HIGH) transfusion threshold for extremely low birth weight infants. J Pediatr 149(3):301-307.e3. 10.1016/j.jpeds.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 10.Robitaille N, Lacroix J, Alexandrov L, Clayton L, Cortier M, Schultz KR, Duval M (2013) Excess of veno-occlusive disease in a randomized clinical trial on a higher trigger for red blood cell transfusion after bone marrow transplantation: a Canadian blood and marrow transplant group trial. Biol Blood Marrow Transplant 19(3):468–473. 10.1016/j.bbmt.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 11.Carson JL, Noveck H, Berlin JA, Gould SA (2002) Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 42(7):812–818. 10.1046/j.1537-2995.2002.00123.x [DOI] [PubMed] [Google Scholar]
- 12.Carson JL, Carless PA, Hébert PC (2012) Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 1–62. 10.1002/14651858.cd002042.pub3 [DOI] [PMC free article] [PubMed]
- 13.Shander A, Javidroozi M, Naqvi S, Aregbeyen O, Çaylan M, Demir S, Juhl A (2014) An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion 54(10pt2):2688–2695. 10.1111/trf.12565 [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet J-P, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ (2007) Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 356(16):1609–1619. 10.1056/nejmoa066240 [DOI] [PubMed] [Google Scholar]
- 15.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 340(6):409–417. 10.1056/nejm199902113400601 [DOI] [PubMed] [Google Scholar]
- 16.Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Hickner A, Rogers MAM (2014) Health care–associated infection after red blood cell transfusion. JAMA 311(13):1317. 10.1001/jama.2014.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacroix J, Demaret P, Tucci M (2012) Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinatol 36(4):225–231. 10.1053/j.semperi.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Jansen AJG, Essink-Bot M, Beckers EAM, Hop WCJ, Schipperus MR, Van Rhenen D (2003) Quality of life measurement in patients with transfusion-dependent myelodysplastic syndromes. Br J Haematol 121(2):270–274. 10.1046/j.1365-2141.2003.04272.x [DOI] [PubMed] [Google Scholar]
- 19.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J (2011) Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 365(26):2453–2462. 10.1056/nejmoa1012452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackritz EM, Hightower AW, Zucker JR, Ruebush TK, Onudi CO, Steketee RW, Were JBO, Patrick E, Campbell CC (1997) Longitudinal evaluation of severely anemic children in Kenya. AIDS 11(12):1487–1494. 10.1097/00002030-199712000-00013 [DOI] [PubMed] [Google Scholar]
- 21.Viele MK, Weiskopf RB (1994) What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah’s witnesses. Transfusion 34(5):396–401. 10.1046/j.1537-2995.1994.34594249050.x [DOI] [PubMed] [Google Scholar]
- 22.Federation of Medical Specialists (2019) Startpagina - Bloedtransfusiebeleid - Richtlijn - Richtlijnendatabase. Federation of Medical Specialists. https://richtlijnendatabase.nl/richtlijn/bloedtransfusiebeleid/startpagina_-_bloedtransfusiebeleid.html. Accessed 14 Jul 2023.
- 23.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Åneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JRM, Nielsen JS, Oldner A, Pettilä V, Cronhjort MB, Andersen LH, Perner A (2014) Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 371(15):1381–1391. 10.1056/nejmoa1406617 [DOI] [PubMed] [Google Scholar]
- 24.Muszynski JA, Guzzetta NA, Hall MW, Macrae D, Valentine SL, Bateman ST, Spinella PC (2018) Recommendations on RBC transfusions for critically ill children with nonhemorrhagic shock from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 19:S121–S126. 10.1097/pcc.0000000000001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henke M, Sindlinger F, Ikenberg H, Gerds T, Schumacher M (2004) Blood hemoglobin level and treatment outcome of early breast cancer. Strahlenther Onkol 180(1):45–51. 10.1007/s00066-004-1123-7 [DOI] [PubMed] [Google Scholar]
- 26.Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, Bentzen J, Overgaard J (2011) The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy—results from the randomized DAHANCA 5 study. Radiother Oncol 98(1):28–33. 10.1016/j.radonc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 27.Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, Grau C, Johansen J, Bentzen J, Andersen L, Evensen JF, Overgaard J (2011) Does transfusion improve the outcome for HNSCC patients treated with radiotherapy?—results from the randomized DAHANCA 5 and 7 trials. Acta Oncol 50(7):1006–1014. 10.3109/0284186x.2011.592650 [DOI] [PubMed] [Google Scholar]
- 28.Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, Chin A, Takes RP, de Bree R, Hoogsteen IJ, Bussink J, Span PN, Kaanders JH (2014) Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer. Clin Cancer Res 20(5):1345–1354. 10.1158/1078-0432.ccr-13-1730 [DOI] [PubMed] [Google Scholar]
- 29.Patient Blood Management Guidelines: Module 3 (2012) Patient Blood Management Guidelines National Blood Authority. https://www.blood.gov.au/pubs/pbm/module3/abbreviations_and_acronyms.html. Accessed 14 Jul 2023.
- 30.Willems A, Harrington K, Lacroix J, Biarent D, Joffe AR, Wensley D, Ducruet T, Hébert PC, Tucci M (2010) Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med 38(2):649–656. 10.1097/ccm.0b013e3181bc816c [DOI] [PubMed] [Google Scholar]
- 31.Hajjar LA, Vincent J-L, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, Fukushima J, Filho RK, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NAG, Auler JOC (2010) Transfusion requirements after cardiac surgery. JAMA 304(14):1559. 10.1001/jama.2010.1446 [DOI] [PubMed] [Google Scholar]
- 32.Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB (2011) Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy*. Pediatr Crit Care Med 12(1):39–45. 10.1097/pcc.0b013e3181e329db [DOI] [PubMed] [Google Scholar]
- 33.Valentine SL, Bembea MM, Muszynski JA, Cholette JM, Doctor A, Spinella PC, Steiner ME, Tucci M, Hassan NE, Parker RI, Lacroix J, Argent A, Carson JL, Remy KE, Demaret P, Emeriaud G, Kneyber MCJ, Guzzetta N, Hall MW, Bateman ST (2018) Consensus recommendations for RBC transfusion practice in critically ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 19(9):884–898. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6126913/pdf/nihms966887.pdf32.332. Accessed 14 Jul 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giammarco S, Chiusolo P, Piccirillo N, Di Giovanni A, Metafuni E, Laurenti L, Sica S, Pagano L (2016) Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol 10(2):147–154. 10.1080/17474086.2017.1270754 [DOI] [PubMed] [Google Scholar]
- 35.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM, Schwartz GEJ (2019) Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special Issue. J Clin Apheresis 34(3):171–354. 10.1002/jca.21705 [DOI] [PubMed] [Google Scholar]
- 36.Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, Keir AK, DenHollander N, Dzik WS, Callum J (2015) A systematic review of transfusion-associated graft-versus-host disease. Blood 126(3):406–414. 10.1182/blood-2015-01-620872 [DOI] [PubMed] [Google Scholar]
- 37.Paul DA, Leef KH, Locke RG, Stefano JL (2002) Transfusion volume in infants with very low birth weight: a randomized trial of 10 versus 20 mL/kg. J Pediatr Hematol Oncol 24(1):43–46. 10.1097/00043426-200201000-00012 [DOI] [PubMed] [Google Scholar]
- 38.Khodabux CM, Hack KEA, von Lindern JS, Brouwers H, Walther FJ, Brand A (2009) A comparative cohort study on transfusion practice and outcome in two Dutch tertiary neonatal centres. Transfus Med 19(4):195–201. 10.1111/j.1365-3148.2009.00934.x [DOI] [PubMed] [Google Scholar]
- 39.Wong H, Connelly R, Day A, Flavin MP (2007) A comparison of high and standard blood transfusion volumes in premature infants. Acta Paediatr 94(5):624–625. 10.1111/j.1651-2227.2005.tb01949.x [DOI] [PubMed] [Google Scholar]
- 40.Olupot-Olupot P, Engoru C, Thompson J, Nteziyaremye J, Chebet M, Ssenyondo T, Dambisya CM, Okuuny V, Wokulira R, Amorut D, Ongodia P, Mpoya A, Williams TN, Uyoga S, Macharia A, Gibb DM, Walker AS, Maitland K (2014) Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia. BMC Med 12(1):67. 10.1186/1741-7015-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (DOCX 9 KB)
Supplementary file2 (DOCX 7 KB)
Supplementary file3 (DOCX 10 KB)
Supplementary file4 (DOCX 103 KB)
Supplementary file5 (DOCX 19.2 KB)
Supplementary file6 (DOCX 755 KB)
Supplementary file7 (DOCX 81.6 KB)
Supplementary file8 (DOCX 19 KB)
Supplementary file9 (DOCX 753 KB)
Supplementary file10 (DOCX 7 KB)
Supplementary file11 (DOCX 455 KB)
Data Availability Statement
No datasets were generated or analyzed during the current study.