Abstract
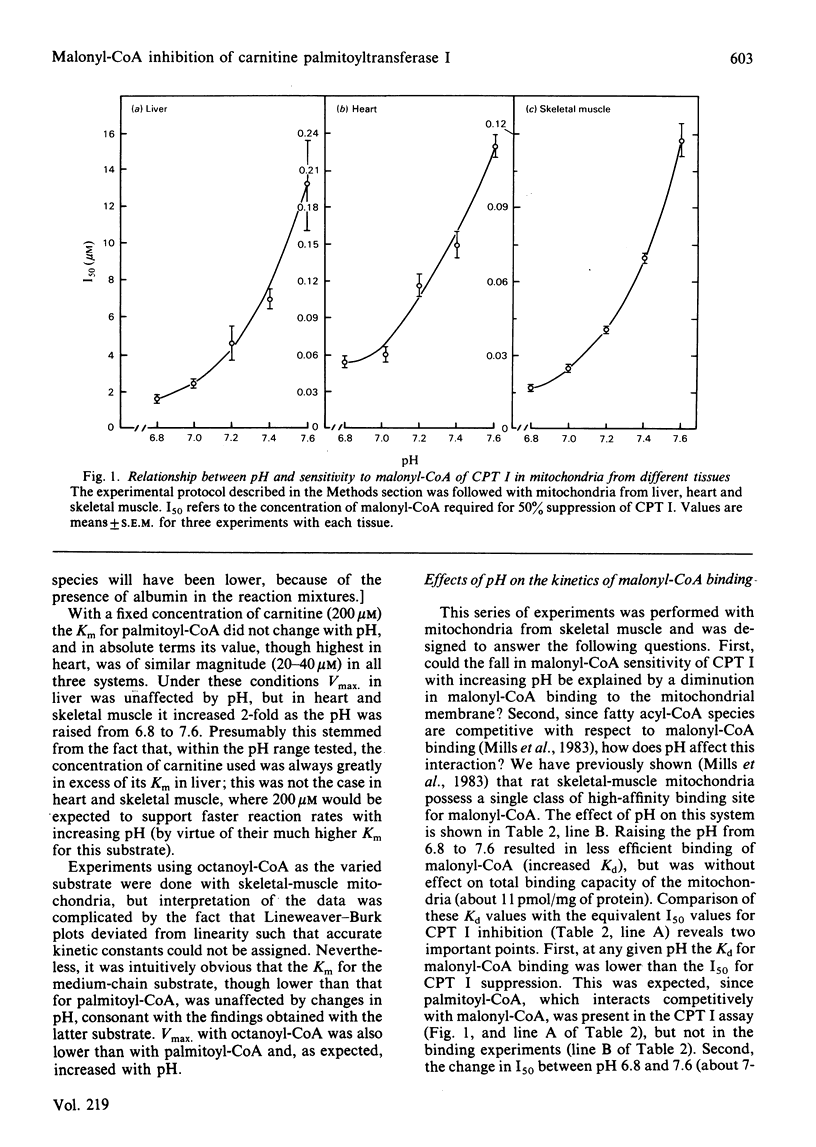
The kinetics of carnitine palmitoyltransferase I (CPT I; EC 2.3.1.21) were examined in mitochondria from rat liver, heart and skeletal muscle as a function of pH over the range 6.8-7.6. In all three tissues raising the pH resulted in a fall in the Km for carnitine, no change in the Km for palmitoyl-CoA or Octanoyl-CoA, and a marked decrease in the inhibitory potency of malonyl-CoA. Studies with skeletal-muscle mitochondria established that increasing pH was accompanied by an increase in the Kd of the malonyl-CoA binding site for this ligand, coupled with a decrease in the Kd for fatty acyl-CoA species to compete for malonyl-CoA binding. Three principal conclusions are drawn. (1) The pH-induced shift in malonyl-CoA sensitivity of CPT I is not a phenomenon restricted to liver mitochondria. (2) At any given pH within the range tested, the ability of malonyl-CoA (and closely related compounds) to inhibit enzyme activity is governed by the efficiency of their binding to the malonyl-CoA site. (3) The competitive interaction between fatty acyl-CoA substrates and malonyl-CoA as regards CPT I activity is exerted at the malonyl-CoA binding site. Finally, the possibility is strengthened that the malonyl-CoA binding site is distinct from the active site of CPT I.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]


