Abstract
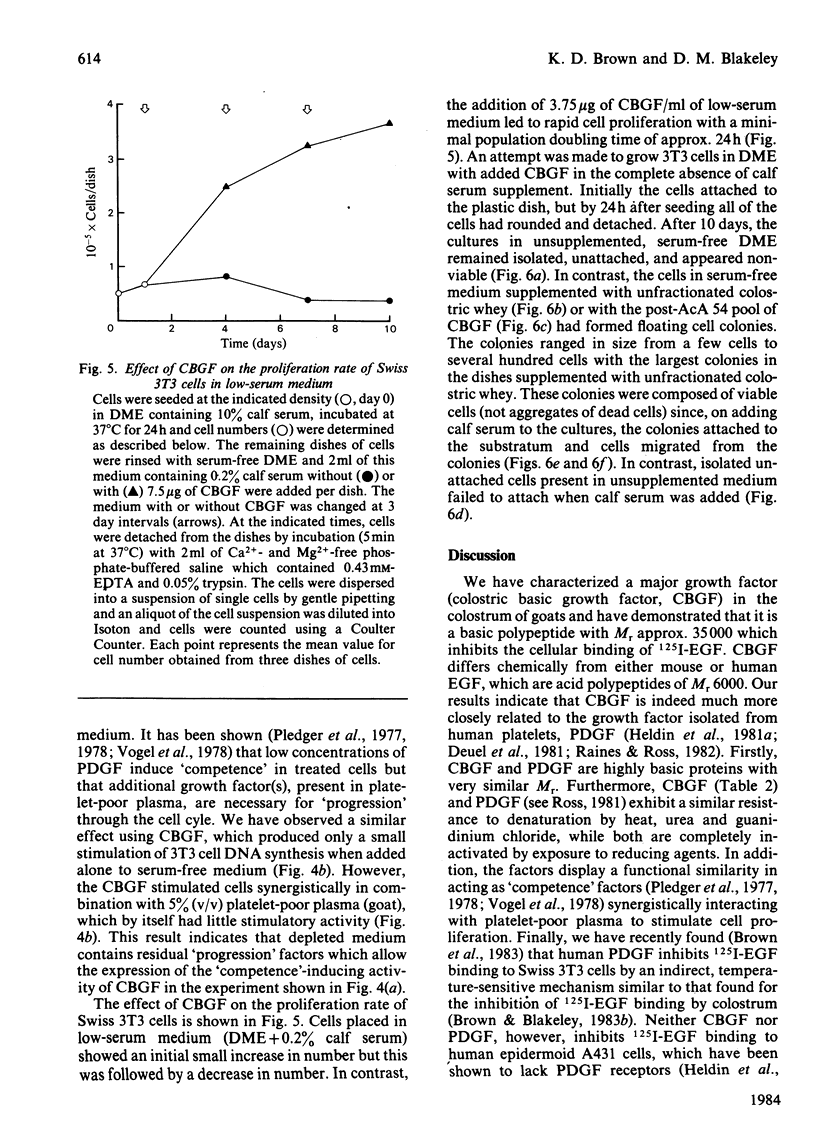
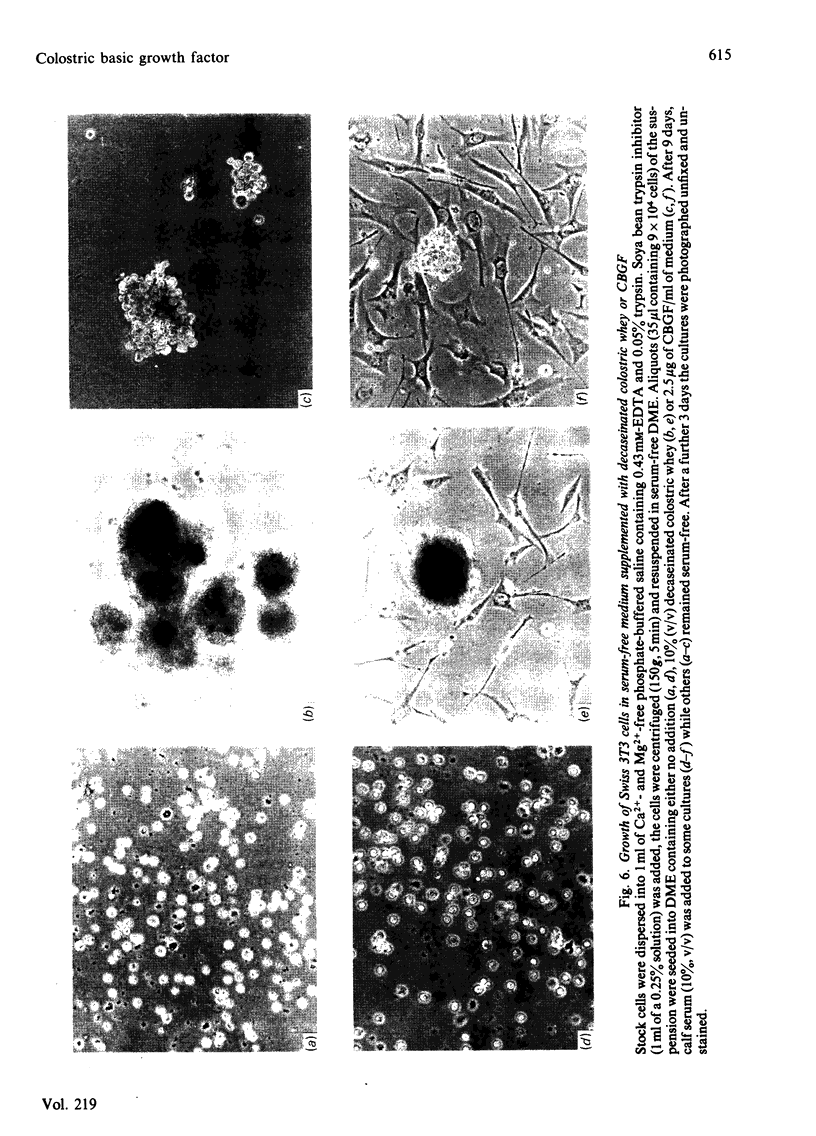
A factor in goat's colostrum which stimulates DNA synthesis and cell proliferation in Swiss 3T3 fibroblasts has been purified approx. 350-fold by a sequence of acid precipitation, cation-exchange chromatography and gel filtration. The growth factor is a highly basic, heat stable (100 degrees C for 5 min) polypeptide with Mr approx. 35000. The polypeptide resists denaturation by guanidinium chloride or urea but is totally inactivated by treatment with reducing agents. The factor, which we have termed colostric basic growth factor ( CBGF ), inhibits the binding of 125I-labelled epidermal growth factor (125I-EGF) to Swiss 3T3 fibroblasts but does not inhibit 125I-EGF binding to epidermoid A431 cells. CBGF interacts synergistically with plasma in stimulating DNA synthesis in quiescent Swiss 3T3 cells. The chemical and biological properties of CBGF are thus very similar to the properties reported for the human platelet-derived growth factor. Although high concentrations of CBGF are present in the colostrum of goats, cows, and sheep, the milk of these species contains little or no factor. The origin and possible functions of CBGF are unknown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardmore J. M., Richards R. C. Concentrations of epidermal growth factor in mouse milk throughout lactation. J Endocrinol. 1983 Feb;96(2):287–292. doi: 10.1677/joe.0.0960287. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Cell growth-promoting activity in mammary secretions of the goat, cow and sheep. Br Vet J. 1983 Jan-Feb;139(1):68–78. doi: 10.1016/s0007-1935(17)30594-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Inhibition of the binding of 125I-labelled epidermal growth factor to mouse cells by a mitogen in goat mammary secretions. Biochem J. 1983 May 15;212(2):465–472. doi: 10.1042/bj2120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., MacDonald M. Inhibition of epidermal growth factor binding to Swiss 3T3 cells by human platelet release-products. Biosci Rep. 1983 Jul;3(7):659–666. doi: 10.1007/BF01172876. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Epidermal growth factor is a major growth-promoting agent in human milk. Science. 1980 Oct 10;210(4466):198–199. doi: 10.1126/science.6968093. [DOI] [PubMed] [Google Scholar]
- Chernoff A., Levine R. F., Goodman D. S. Origin of platelet-derived growth factor in megakaryocytes in guinea pigs. J Clin Invest. 1980 Apr;65(4):926–930. doi: 10.1172/JCI109747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Concentrations of epidermal growth factor, nerve growth factor, and submandibular gland renin in male and female mouse tissue and fluids. Endocrinology. 1979 Dec;105(6):1382–1387. doi: 10.1210/endo-105-6-1382. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Epidermal growth factor (urogastrone) in human fluids: size heterogeneity. J Clin Endocrinol Metab. 1979 Apr;48(4):673–679. doi: 10.1210/jcem-48-4-673. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Imagawa W., Tomooka Y., Nandi S. Serum-free growth of normal and tumor mouse mammary epithelial cells in primary culture. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4074–4077. doi: 10.1073/pnas.79.13.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Ruggiero J. T., Leung L. K., Doyle M. J., McKeown-Longo P. J., Mosher D. F. Cultured human fibroblasts synthesize and secrete thrombospondin and incorporate it into extracellular matrix. Proc Natl Acad Sci U S A. 1983 Feb;80(4):998–1002. doi: 10.1073/pnas.80.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Chao F. C., Stiles C. D., Antoniades H. N., Scher C. D. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979 Jun;53(6):1043–1052. [PubMed] [Google Scholar]
- Klagsbrun M. Bovine colostrum supports the serum-free proliferation of epithelial cells but not of fibroblasts in long-term culture. J Cell Biol. 1980 Mar;84(3):808–814. doi: 10.1083/jcb.84.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Doyle M. J., Jaffe E. A. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982 May;93(2):343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Singh J. P., Chaikin M. A., Stiles C. D. Phylogenetic analysis of platelet-derived growth factor by radio-receptor assay. J Cell Biol. 1982 Nov;95(2 Pt 1):667–671. doi: 10.1083/jcb.95.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Klagsbrun M. Serum-free growth of normal and transformed fibroblasts in milk: differential requirements for fibronectin. J Cell Biol. 1981 Feb;88(2):294–300. doi: 10.1083/jcb.88.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]