Abstract
Autoimmune encephalitis (AE) is an immune-mediated condition that induces brain inflammation due to several neural-specific autoantibodies. The main triggering and predisposing factors are infections, genetics, the use of immune checkpoint inhibitors and tumors. We report a case of a 57-year-old male with a biopsy-confirmed Langerhans cell histiocytosis (LCH) and a concomitant anti-LGI1 encephalitis discussing a possible relationship in the pathogenesis of these phenomena. Only sporadic cases of AE developing in the context of histiocytic neoplasms have been described and there are no other reports on the relationship between LGI1-AE and LCH worldwide due to small number of cases.
Keywords: LGI1 encephalitis, Langerhans cell histiocytosis, IL-6, Paraneoplastic, Case report
Highlights
-
•
LCH is defined as “inflammatory myeloid neoplasm” due to both inflammatory and neoplastic characteristics.
-
•
The cytokine storm involved in LCH could play as a trigger for an autoimmune response leading to anti-LGI1 encephalitis.
-
•
Anti-LGI1 encephalitis could be a paraneoplastic manifestation of LCH.
1. Introduction
Autoimmune encephalitis (AE) includes a heterogeneous group of immune-mediated inflammatory brain disorders, potentially reversible and with a good clinical outcome if promptly recognized and treated [1]. In the last decade different entities were described through the identification of several neural-specific autoantibodies directed against key proteins for cellular functions, either intracellular or located on the neuronal surface (Neuronal surface antibodies, NSAbs). If the formers are non-pathogenic and related to a T-mediated immunity against a neoplasm with secondary response against nervous system, NSAbs are likely pathogenic and are less associated with neoplasms [2]. Among them, antibodies against N-methyl-D-aspartate receptor (NMDAR) and anti-leucine-rich glioma-inactivated 1 (LGI1) have been reported in most cases of AE.
Anti-LGI1 encephalitis has a median age of onset of about 60 years and typically involves limbic areas with rapidly progressive cognitive decline, memory and visuospatial impairment, behavioral changes, faciobrachial dystonic seizures, sleep and autonomic disturbances, and hyponatremia due to a syndrome of inappropriate antidiuretic hormone secretion (SIADH) [3,4].
The four currently recognized AE triggering and predisposing factors are infections, genetics, the use of immune checkpoint inhibitors and tumors [5].
In anti-LGI1 AE only <10 % of cases are related to lymphoma, thymoma, thyroid and breast tumors [6] and SCLC [7]; the other cases are non-paraneoplastic forms.
Sporadic cases of AE developing in the context of histiocytic neoplasms have been reported.
Histiocytic neoplasms, a rare and heterogeneous group of disorders, primarily include Erdheim-Chester disease, Langerhans cell histiocytosis, and Rosai-Dorfman disease. Langerhans cell hystiocytosis (LCH) is a clonal disorder of dendritic cells [8] infiltrating endocrine (50–70 %), bones (60 %) and respiratory (50–60 %) tissues, lymph nodes, and dura often extending from calvarium. Frequently, skin is also involved with papular rash, subcutaneous nodules, or xanthelasma-like lesions [9].
LCH has been associated with other autoimmune processes, including membranous nephropathy [10], systemic lupus erythematosus [11], autoimmune haemolytic anemia [12], Evans syndrome [13], autoimmune hepatitis [14].
We report a case of a 57-year-old male with a biopsy-confirmed Langerhans cell histiocytosis and a concomitant anti-LGI1 encephalitis discussing a possible relationship in the pathogenesis of these phenomena.
2. Case presentation
In spring 2021, a 57-year-old male with hypercholesterolemia and cluster headache presented with involuntary movements of the left upper limb and episodes of epigastric sensation with irradiation up to the cervical area. These episodes lasted about 3–4 seconds and were repeated about 4–5 times a day. Attention and memory deficits were also added. During the summer his family reported increased appetite, change in food tastes and dysphoria characterized by social inadequacy. For this reason, he was initially followed by a psychiatrist, who prescribed valproic acid which determined a clear reduction in the frequency of epigastric paroxysmal episodes. In October 2021, he underwent a brain magnetic resonance imaging (MRI) which revealed a T2/FLAIR hyperintensity and swelling of right amygdala and hippocampus, without contrast enhancement or restricted diffusion. For this reason, the patient was admitted to Neurological Department, where several investigations were carried out. He performed an EEG and a sleep deprived EEG, both of which yielded normal results. Cerebrospinal fluid (CSF) analysis revealed mild pleocytosis (9 cells/μL) and hyperproteinorrachia (580 mg/L). Furthermore, neuronal surface antibodies were tested on CSF and serum sample through indirect immunofluorescence on transfected EU 90 cells (“Euroimmun”). Test for anti-leucine-rich glioma-inactivated 1 (LGI1) antibodies was positive both on CSF and serum (with a titre of 1:10 on serum on the latter), consistent with a diagnosis of LGI-AE.
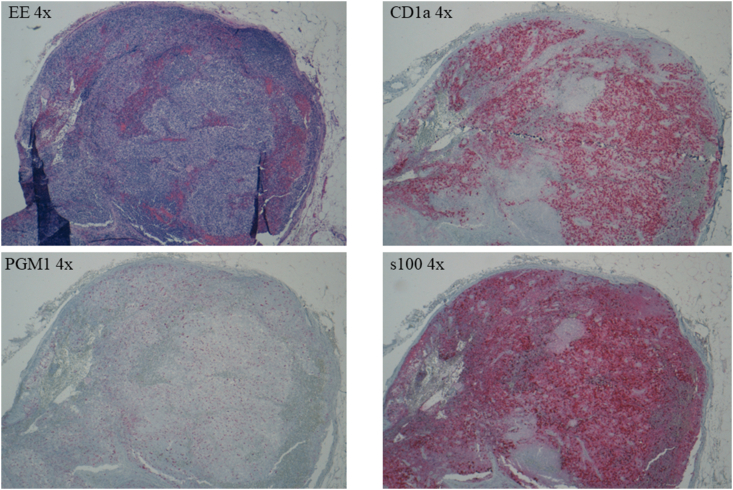
To rule out the underlying presence of neoplasia, the patient underwent total body Computed Tomography (CT) that showed left inguinal lymphadenomegaly. This finding led to the performance of an excisional biopsy of the left inguinal lymph node, resulted compatible with LCH, BRAF-V600E negative. Specifically, these lymph node slides showed altered architecture with the presence of proliferation consisting predominantly of medium-sized epithelioid elements, with round, broad and weakly eosinophilic cytoplasm, elongated or curved nucleus, sometimes "reniform" and frequently with basophilic or eosinophilic nucleolus. These elements showed expression of CD1a and s100 and nonexpression of CD68/PG-M1 (expressed in rare, fractionated macrophages) and CD21. Proliferation has a sinus and paracortical distribution, and scattered eosinophilic granulocytes and small lymphocytes with regular nuclei are present; there is no evidence of macrophages with foamy cytoplasm and/or capturing pigment. No evidence of necrosis. Conserved distribution of B (CD20+, CD5−) and T (CD3+, CD5+) lymphocyte populations. Expression of Bcl-6 limited to germinal centers present, where there is no clear expression of Bcl-2, in the presence of high growth fraction (ki-67) as per reactive centers. Not significant expression of cyclin-D1 molecule in B-lymphocyte elements (as shown in Fig. 2).
Fig. 2.
EE 4x: hematoxylin and eosin, original magnification 4x
CD1a 4x: immunostaining with CD1a antibody, original magnification 4x
PGM1 4x: immunostaining with CD68/PGM1 antibody, original magnification 4x
s100 4x: immunostaining with protein S-100, original magnification 4x
First-line immunotherapy with methylprednisolone (1 g/day) was administered for 5 days, followed by IVIG (0.4 g/Kg/day) for 5 consecutive days with behavioral improvement and no adverse events. To better control seizures, carbamazepine was added to other AEDs, getting the disappearance of epigastric aura.
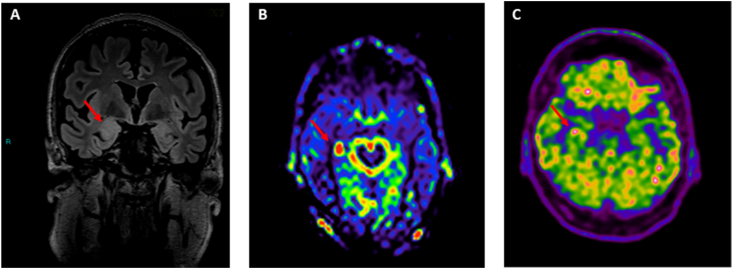
The patient was discharged with a diagnosis of LCH (BRAF-V600E negative) and anti-LGI1 encephalitis. Two months later, he came to our Center for a second opinion on these two diagnoses. Anti-LGI1 antibodies were retested on serum and were still positive (titre 1:10). A brain MRI was repeated showing right temporo-mesial abnormalities compatible with limbic AE; arterial spin labeling sequences also indicated a hyperperfusion of right mesial temporal area (as shown in Fig. 1 – A, B). For the first time, a total body FDG-Positron Emission Tomography (PET) was performed showing hypermetabolism in right mesial temporal area (as shown in Fig. 1 – C), consistent with the diagnosis of limbic AE, and no other areas of hypermetabolism in the rest of the body. In addition, several lymph node slides were sent to us for a second histological examination, which confirmed the diagnosis of LCH.
Fig. 1.
– A, B, C
A: Brain T2 FLAIR MRI: red narrow shows hyperintensity and swelling of right amygdala and hippocampus
B: Arterial spin labelling (ASL) sequence: red narrow shows a hyperperfusion of right mesial temporal area
C:Brain FDG-PET: red narrow shows hypermetabolism in right mesial temporal area
The patient underwent hematologic evaluation which suggested other investigations to better characterized the systemic extension of LCH, such as hormonal screening, skin punch biopsy and a chest Computed Tomography. All these analyses excluded the need of further therapies because there were no other localizations of LCH.
The patient was discharged in good conditions with prednisone 37.5 mg/day and an indication to taper steroid therapy. At 6-month follow-up visit, he was stable, with no seizures and no memory impairment.
3. Discussion
LGI1-antibodies are the second most detected antibodies in autoimmune encephalitis after anti-NMDAR [15].
The pathogenesis is B cell/plasma cell mediated and induced by NSAbs. As these antibodies persist during the time, augmentation of autoreactive B cells, which release autoantigens, plays a role in perpetuating autoimmune neuroinflammation [16] and increasing the risk of systemic immune activation.
From the review of the literature, we only found one case of autoimmune encephalitis linked to histiocytic neoplasms. It is a child with LCH who developed sleep disorder with involuntary movements with a finding of anti-IgLON family protein 5 (IgLON5) antibodies [17].
Focusing on LCH, it is designated as “inflammatory myeloid neoplasm” because of its inflammatory and neoplastic features. LCH cells carrying an oncogenic mutation are hypothesized to proliferate and migrate to the site of injury, recruiting and activating various inflammatory cells [18].
The main oncogenic mutations described are BRAF-V600E and MAP2K1, although their frequency in LCH ranges from 38 % to 57 % [[19], [20], [21]]. In our case, we did not find the BRAF-V600E mutation.
As an inflammatory myeloid neoplasm, hypercytokinemia plays an important role in the pathogenesis of LCH, as reflected in biopsy and serological studies. The former shows infiltrating T cells and profound microglia activation and the latter higher serum levels of cytokines and chemokines. Either IL-8, IL-9, IL-10, IL-12 and an imbalance of IL-2, IL-6, and T-helper-2 cells have been described [14,22,23].
Furthermore, many studies [[24], [25], [26], [27]] emphasize the role of interleukine 6 (IL-6), a cytokine produced by dendritic cells. This cytokine plays a key role in the differentiation of B cells into plasma cells, increases plasmablasts survival thus affecting antibody production [28]. In addition, IL-6 increases the permeability of the blood-brain barrier, induces the differentiation of CD4+ naive T helper cells into Th 17 cells, and inhibits the differentiation of these cells into Treg cells. These changes result in a Treg/Th 17 imbalance, which is believed to be an important pathological mechanism for the development of autoimmune diseases, such as AE [29,30].
Under pathological conditions, such as inflammation, dendritic cells (DCs) can be recruited into brain tissue [31], carrying antigens from the CNS into the regional lymph nodes and triggering specific immune responses [32,33].
Considering these elements, in our patient, we can speculate that the cytokine storm involved in LCH might let DCs in CNS. DCs on the one side could have met LGI1 antigen triggering an autoimmune response, and, on the other, they would have produced the IL-6 increasing the B-cells activity. According to this possible pathophysiological mechanism, the anti LGI1 encephalitis could be a paraneoplastic manifestation of underlying LCH. However, in our patient, cytokine expression was very localized making distant brain involvement less probable and co-occurrence of both diseases more likely. Lastly, there is no report on the relationship between LGI1-AE and LCH worldwide due to small number of cases and we need further studies that can strengthen the idea of a possible relationship between these two clinical entities.
4. Conclusions
In rare diseases, such as autoimmune encephalitis, it is important to always investigate possible inflammatory triggers and/or look for neoplasia.
Patient Perspective. Our patient was relieved when informed that his neurological disease was treatable and that LCH was localized only to the lymph node. He perceived a prompt improvement in his condition after IVIg therapy. Eventually, he was thankful to the neurologists who had cured him and was glad to agree to share his case, understanding that he had had a very rare condition.
CRediT authorship contribution statement
Denise Cerne: Writing – original draft. Federico Massa: Conceptualization. Marco Mora: Supervision, Investigation. Silvia Morbelli: Investigation. Luca Roccatagliata: Investigation. Giacomo Rebella: Investigation. Flavio Villani: Investigation. Federica Bozzano: Investigation. Antonio Uccelli: Supervision. Luana Benedetti: Writing – review & editing, Supervision. Corrado Cabona: Writing – review & editing, Supervision.
Author declaration
All authors participated in the conception and design, drafting the article, or revising it critically for important intellectual content. All authors approved the final version of the article to be submitted.
Ethics statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Data availability statement
Data associated with this case has not been deposited into a publicly available repository and will be made available upon request.
Funding
Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – (DN. 1553 11.10.2022).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abboud H., et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry. Jul. 2021;92(7):757–768. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J. Clin. Neurol. 2016;12(1):1. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Sonderen A., et al. Anti-LGI1 encephalitis. Neurology. Oct. 2016;87(14):1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 4.Goodfellow J.A., Mackay G.A. Autoimmune encephalitis. J. Roy. Coll. Phys. Edinb. Dec. 2019;49(4):287–294. doi: 10.4997/jrcpe.2019.407. [DOI] [PubMed] [Google Scholar]
- 5.Vogrig A., Muñiz-Castrillo S., Desestret V., Joubert B., Honnorat J. Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors. Ther Adv Neurol Disord. Jan. 2020;13 doi: 10.1177/1756286420932797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellul M.A., Wood G., Van Den Tooren H., Easton A., Babu A., Michael B.D. Update on the diagnosis and management of autoimmune encephalitis. Clin. Med. Jul. 2020;20(4):389–392. doi: 10.7861/clinmed.2020-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z., Cui T., Shi W., Wang Q. Clinical analysis of leucine-rich glioma inactivated-1 protein antibody associated with limbic encephalitis onset with seizures. Medicine. Jul. 2016;95(28):e4244. doi: 10.1097/MD.0000000000004244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama H.A., et al. Highlights of the management of adult histiocytic disorders: langerhans cell histiocytosis, erdheim-chester disease, rosai-dorfman disease, and hemophagocytic lymphohistiocytosis. Clin. Lymphoma, Myeloma & Leukemia. Jan. 2021;21(1):e66–e75. doi: 10.1016/j.clml.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal G., et al. The mayo clinic histiocytosis working group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms: erdheim-chester disease, langerhans cell histiocytosis, and rosai-dorfman disease. Mayo Clin. Proc. Oct. 2019;94(10):2054–2071. doi: 10.1016/j.mayocp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Rachima C.M., Cohen E., Iaina N.L., Tobar A., Garty M. A case of langerhans’-cell histiocytosis with membranous nephropathy. Am. J. Kidney Dis. Feb. 2004;43(2):e3.1–e3.7. doi: 10.1053/j.ajkd.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Robak T., et al. Langerhans cell histiocytosis in a patient with systemic lupus erythematosus: a clonal disease responding to treatment with cladribine, and cyclophosphamide. Leuk. Lymphoma. Jan. 2002;43(10):2041–2046. doi: 10.1080/1042819021000015998-1. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira M.C.L.A., Oliveira B.M., Murao M., Vieira Z.M., Gresta L.T., Viana M.B. Clinical course of autoimmune hemolytic anemia: an observational study. J. Pediatr. Mar. 2006;82(1):58–62. doi: 10.2223/JPED.1438. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji Y., Kogawa K., Imai K., Kanegane H., Fujimoto J., Nonoyama S. Evans syndrome in a patient with Langerhans cell histiocytosis: possible pathogenesis of autoimmunity in LCH. Int. J. Hematol. Jan. 2008;87(1):75–77. doi: 10.1007/s12185-007-0007-x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A., Ali H., Galan M., Jiang J., Lingiah V. Concurrent langerhans cell histiocytosis and autoimmune hepatitis: a case and review of the literature. Cureus. Nov. 2020 doi: 10.7759/cureus.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Sonderen A., Petit-Pedrol M., Dalmau J., Titulaer M.J. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. May 2017;13(5):290–301. doi: 10.1038/nrneurol.2017.43. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos H., Dalakas M.C. The immunobiology of autoimmune encephalitides. J. Autoimmun. Nov. 2019;104 doi: 10.1016/j.jaut.2019.102339. [DOI] [PubMed] [Google Scholar]
- 17.Ye F., Fan C., Peng M., Liu S., Yu Y., Yang L. Anti-IgLON5 disease in a pediatric patient with Langerhans cell histiocytosis. Clin. Chim. Acta. Oct. 2021;521:212–214. doi: 10.1016/j.cca.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Berres M.-L., Allen C.E., Merad M. 2013. Pathological Consequence of Misguided Dendritic Cell Differentiation in Histiocytic Diseases; pp. 127–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badalian-Very G., et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. Sep. 2010;116(11):1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haroche J., et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. Sep. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M., Tojo A. Langerhans cell histiocytosis in adults: advances in pathophysiology and treatment. Cancer Sci. Dec. 2018;109(12):3707–3713. doi: 10.1111/cas.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grois N. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain. Feb. 2005;128(4):829–838. doi: 10.1093/brain/awh403. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto A., et al. Inflammatory serum cytokines and chemokines increase associated with the disease extent in pediatric Langerhans cell histiocytosis. Cytokine. Sep. 2017;97:73–79. doi: 10.1016/j.cyto.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Byun J.-I., et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. J. Neuroimmunol. Aug. 2016;297:141–147. doi: 10.1016/j.jneuroim.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Dale R.C. Interleukin-6 blockade as rescue therapy in autoimmune encephalitis. Neurotherapeutics. Oct. 2016;13(4):821–823. doi: 10.1007/s13311-016-0471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W.-J., et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. Oct. 2016;13(4):824–832. doi: 10.1007/s13311-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smets I., Titulaer M.J. Antibody therapies in autoimmune encephalitis. Neurotherapeutics. Apr. 2022;19(3):823–831. doi: 10.1007/s13311-021-01178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jego G., Palucka A.K., Blanck J.-P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. Aug. 2003;19(2):225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee G. The balance of Th17 versus Treg cells in autoimmunity. Int. J. Mol. Sci. Mar. 2018;19(3):730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng C., et al. Th17 cells regulate the progress of anti-NMDAR encephalitis. Am J Transl Res. 2022;14(9):6268–6276. [PMC free article] [PubMed] [Google Scholar]
- 31.Serafini B., Columba-Cabezas S., Di Rosa F., Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am. J. Pathol. Dec. 2000;157(6):1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vos A.F., et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J. Immunol. Nov. 2002;169(10):5415–5423. doi: 10.4049/jimmunol.169.10.5415. [DOI] [PubMed] [Google Scholar]
- 33.Karman J., Ling C., Sandor M., Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J. Immunol. Aug. 2004;173(4):2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this case has not been deposited into a publicly available repository and will be made available upon request.