Abstract
The thyroid eye disease (TED) of Graves disease is associated with high titers of stimulating TSH receptor antibodies, retro-orbital inflammation, fibroblast release of cytokines and chemokines, and adipogenesis, which in turn leads to proptosis, muscle fibrosis, and dysfunction. Part of this scenario is the induction of fibroblast proliferation and autophagy secondary to synergism between the TSH receptor (TSHR) and the insulin-like growth factor-1 receptor (IGF-1R). While TED is well associated with thyroid-stimulating antibodies to the TSHR, which is also well expressed on fibroblasts, in fact the TSHR reactome has a variety of TSHR antibodies with varying biological activity. Therefore, we have now evaluated the possible role of neutral TSHR antibodies (N-TSHR-mAbs), directed at the hinge region of the TSHR, which do not induce cell proliferation but are known to have effects on multiple proteins in thyroid cells including stress-related signaling molecules. We examined the consequences of an N-TSHR-mAb acting on TSHR-expressing fibroblasts and found marked cell stress, which initiated signaling pathways involving inflammasome activation. This response ended in widespread cell death by pyroptosis through activation of caspase 8 and gasdermin D. Hence, not only can stimulating TSHR autoantibodies influence TED inflammation but the N-TSHR antibodies, representing more of the reactome, may also exaggerate the retro-orbital inflammatory response seen in TED.
Keywords: thyroid eye disease, Graves disease, TSH receptor antibody
As part of our analysis of mechanisms in thyroid eye disease (TED), we recently reported the role of stimulating thyrotropin receptor antibodies (TSHR-Abs) in inducing fibroblast proliferation and adipogenesis and enhancing autophagy, actions that synergized with the action of the insulin-like growth factor-1 receptor (IGF-1R) [1-4]. Furthermore, we showed that a monoclonal antibody blocking the IGF-1R inhibited such actions and led to apoptosis of fibroblasts and suggested this as an important mechanism in the successful clinical application of such an antibody in TED [5]. The stimulating TSHR antibodies are part of a reactome that includes antibodies of differing biological action. In the present report we investigated the action on fibroblasts of a type of TSHR-Ab that interacts with the linker-region of the TSHR, often called the hinge region, and that do not induce traditional TSHR signal transduction and hence the common term “neutral” TSHR-Abs (N-TSHR-mAbs) [6].
We have shown previously that these N-TSHR-Abs induce intense thyroid cell stress that leads to excess intracellular reactive oxygen species (ROS) accumulation in the setting of no traditional TSHR signaling, including no cyclic adenosine monophosphate (cAMP) accumulation [7]. Our studies to determine the mechanisms of ROS induction first disclosed there were multiple endoplasmic reticulum (ER) stress markers and misfolded protein markers induced [8]. The ER is a crucial location for protein folding and maturation and the unfolded protein response (UPR) signal includes both ER stress and the production of ROS, which would suggest UPR involvement following N-TSHR-Ab endocytosis [9]. Since mitochondria are the major site for ROS induction, N-TSHR-Abs induced mitochondrial ROS leading to increased ER stress, and this in turn compromised UPR function. Furthermore, the increased ROS levels also disrupted additional intracellular organelle integrity, such as the lysosomes, causing them to also fail to function properly [9, 10]. ROS may react with and destroy a variety of biological macromolecules such as DNA, proteins, and lipids, so normal ROS production is tightly regulated within cells and such redox agents can act as signaling molecules in a variety of cell-signaling pathways. Our findings with thyroid cells clearly showed that ROS generated by endocytosis of N-TSHR-mAbs/TSHR complexes triggered multiple signaling cascades that resulted in multiorganelle damage and thyroid cell death.
As opposed to thyroid cells, it is TSHR-expressing fibroblasts that appear to be most involved in the pathogenesis of TED. Our results now define some of the signals generated by fibroblast TSHRs in response to N-TSHR-mAb exposure and show that fibroblasts undergo a comparable degree of cell stress as thyroid cells do when exposed to an N-TSHR monoclonal antibody. When fibroblast cells die by pyroptosis, they trigger an inflammatory response from activated inflammasome signals leading to the local immunological reactions seen in the retro-orbital tissues in TED [11, 12].
Materials and Methods
Cell Culture and Treatments
To synchronize all cells to the same cell cycle phase and basal levels of signaling molecules, 3T3 L mouse fibroblasts were starved for 2 days and cultured as described previously [6]. Before any stimulation experiments, cells were made quiescent by starvation in bovine calf serum free basal medium (Dulbecco’s modified Eagle’s medium [DMEM]) containing 0.3% (wt/vol) bovine serum albumin for 2 days [13]. Before stimulation with N-TSHR-mAbs (mouse neutral monoclonal antibody—MC1; IgG2) (Table 1 provides all antibody details), cells were fixed and permeabilized for In-Cell Western analysis.
Table 1.
Antibodies used in the described methods
| Name | Host | Source | Catalog No. | ∼Dilution | |||
|---|---|---|---|---|---|---|---|
| ICC | ICW | WB | RRID | ||||
| IGF-1R-B-mAb, 1H7 IgG1 κ | Mouse | Santa Cruz Biotechnology Inc | SC461 | — | — | AB_671786 | |
| TSHR-St-mAb, M22 | Human | RSR Ltd | — | — | — | AB_2892140 | |
| TSHR-St-mAb, MS1 | Hamster | Davies Laboratory | — | — | — | AB_3552210 | |
| pMek1/2-Ab (S217/221) | Rabbit | Cell Signaling Technology | 9121 | — | 1:100 | 1:1000 | AB_331648 |
| pErk1/2-Ab (T202/Y204) | Rabbit | Cell Signaling Technology | 4370 | — | 1:100 | 1:1000 | AB_2315112 |
| pAkt-Ab (S473) | Rabbit | Cell Signaling Technology | 4060 | — | 1:100 | 1:1000 | AB_ 2315049 |
| pPI3K, p85 (Y458) | Rabbit | Cell Signaling Technology | 3821 | — | 1:100 | 1:1000 | AB_330320 |
| Mek1/2 (nonphospho) | Rabbit | Cell Signaling Technology | 8727 | — | 1:100 | 1:1000 | AB_ 10829473 |
| Erk1/2 (nonphopho) | Rabbit | Cell Signaling Technology | 4695 | — | 1:00 | 1:1000 | AB_ 390779 |
| Akt (nonphospho) | Rabbit | Thermo Fisher Scientific | 4685 | — | 1:100 | 1:1000 | AB_2225340 |
| PI3K, p85 (nonphospho) | Rabbit | Cell Signaling Technology | 4257 | — | 1:100 | 1:1000 | AB_ 659889 |
| Cytochrome-C | Rabbit | Cell Signaling Technology | 11940 | — | 1:100 | 1:1000 | AB_2637071 |
| Caspase-3 | Rabbit | Cell Signaling Technology | 14220 | — | 1:100 | 1:1000 | AB_2798429 |
| Caspase-9 | Rabbit | Cell Signaling Technology | 20750 | — | 1:100 | 1:1000 | AB_2798848 |
| Caspase-8 | Rabbit | Aviva Systems Biology Co | OAMA03439 | — | 1:100 | 1:1000 | AB_10873136 |
| FADD | Rabbit | Aviva Systems Biology Co | ARP30294 | — | 1:100 | 1:1000 | AB_938079 |
| pIGF-1R-Ab (Y1161) | Rabbit | Biomatik Co | 10895 | — | 1:100 | 1:1000 | AB_731544 |
| Beclin 1 | Rabbit | St Johns Laboratory | STJ97761 | 1:200 | 1:100 | 1:1000 | — |
| LC3A | Rabbit | St Johns Laboratory | STJ97755 | 1:200 | 1:100 | 1:1000 | — |
| LC3B, α MAP | Rabbit | St Johns Laboratory | STJ97398 | 1:200 | 1:100 | 1:1000 | — |
| ULK1 | Rabbit | Abclonal | A8529 | — | 1:100 | 1:1000 | AB_2772810 |
| P62 | Rabbit | Abclonal | A11483 | — | 1:100 | 1:1000 | AB_2861579 |
| Isotype control, IgG1, k-chain | Mouse | BD Bioscience | 562438 | — | — | AB_11207319 | |
| β-Actin-mAb | Mouse | Sigma Millipore | A1978 | — | — | 1:1000 | Ab_476692 |
Fluorescein isothiocyanate–conjugated goat antirabbit (1:200 dilution, Jackson Laboratory) for immunocytochemistry (ICC), horseradish peroxidase–conjugated antibody (Cell Signaling, 1:12 000) for Western blot (WB), and IRDye anti-rabbit (680/800, 1:800) for In-Cell Western (ICW, Li-Cor Biosciences).
Cyclic Adenosine Monophosphate Generation
3T3 fibroblast (80% confluent) cells in 96-well plates were used for intracellular cAMP levels measured by enzyme immunoassay (Assay Designs Inc, or Amersham cAMP Biotrak EIA System, GE Healthcare Bio-Sciences Corp) using polyclonal antibody to cAMP to bind, in a competitive manner, the cAMP in the standards or samples or a conjugate molecule that had cAMP covalently attached. The intensity of the bound color was inversely proportional to the concentration of cAMP in either standards or samples. In each treatment, 2-mM 3-isobutyl-1-methylxanthine (Sigma) in the basal medium was used for measuring intracellular cAMP levels.
Cell Proliferation Assessment
A cholecystokinin-octapeptide (CCK-8) kit was used to determine cell proliferation according to the instructions provided with the kit (Dojindo). With WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt), which forms a water-soluble formazan dye following bioreduction in the presence of an electron carrier, 1-Methoxy PMS, CCK-8 enables convenient tests. The CCK-8 solution is added straight into the cells. Cellular dehydrogenases bioreduce WST-8 to an orange formazan product that dissolves in tissue culture media. The number of live cells directly correlates with the amount of formazan generated. There is an increase in the sensitivity of detection. Cell-proliferation experiments were performed in a 96-well plate in triplicate in basal medium without any hormones. Briefly, cells (3 × 103 per well) were seeded and maintained in 200-μL medium in a humidified incubator. Cells were then made quiescent by with 0.3% bovine serum albumin for 2 days as described earlier. The colorimetric CCK-8 proliferation assays were performed at day 3 after treatment. The optical density was determined using a spectrophotometer (DTX 880 Multimode Detector; Beckman Coulter) at a wavelength of 570 nm.
Reactive Oxygen Species Detection
Live-cell microscopy was performed for visualization of ROS. Cells were grown in delta T dishes and treated with different antibodies and reagents for 1 hour. The cells were then incubated with dyes for mitochondrial ROS (mROS) (MitoSOX, Thermofisher Inc) and total ROS (tROS) (D123, Enzo Life Sciences) for 15 minutes and then washed 3 times with HBSS buffer and observed under the microscope with Hoechst 33342 nuclear dye in the medium. Similarly, cells grown in 96-well plates were used to label mROS and tROS and analyzed in a fluorescent plate reader for quantitation (Clariostar, BMG). As controls, a protein kinase A (PKA) inhibitor (H89) was purchased from Cayman Chemical Co, and bovine TSH was from Sigma.
Immunocytochemistry
Immunocytochemistry (ICC) was performed according to prescribed protocols (Cell Signaling Technology Inc). Briefly, after washing with phosphate-buffered saline (PBS, pH 7.5), adherent cells on slides were fixed in 4% (wt/vol) paraformaldehyde, permeabilized in 90% methanol (vol/vol), and blocked for 2 hours in blocking buffer. The cells were then incubated overnight at 4 °C with specific antibodies diluted in blocking buffer (see Table 1). After washing, the slides were incubated with fluorescently conjugated secondary antibodies for 1 hour at room temperature with appropriate dilution, washed 3 times in PBS, mounted with mounting medium containing nuclear dye DAPI (4′,6-diamidino-2-phenylindole), and visualized immediately under a digital (Eclipse TE2000-S, Nikon) or confocal microscope (Zeiss LSM 700). At least 5 images were captured per experiment, and 3 images each of treated or untreated cells were analyzed quantitatively by different software. Quantification of live fluorescence imaging and ICC images were loaded into an Image-Pro system for analysis, in which different colors were visually quantified by 2 independent observers. The cells positive for both the target color and nuclear staining (Hoechst 33342) were considered positive for fluorescence and digitally recorded to prevent multiple counts. Fluorescent intensity (FI) was calculated using CCTF (corrected total cell fluorescence) in Image-Pro software: CCTF = integrated density − (area of selected cell × mean fluorescence of background readings).
Confirmation of Signaling Molecules by Immunoblots
Immunoblots were performed as described previously [13]. Briefly, blots were incubated with primary antibodies at 4 °C overnight. After washing they were incubated with horseradish peroxidase–labeled secondary antibody for 1 hour at room temperature, washed thrice with washing buffer, and then visualized [13]. The pathway phospho and nonphospho antibodies were used as listed in Table 1. All antibodies used for ICC, Li-Cor, In-Cell Western (ICW), and Western blot (WB) are also described in Table 1. Rabbit monoclonal antibody against 8-hydroxy-2′-deoxy guanosine (8-OHdG) was used to detect oxidative DNA damage.
Proteomic Arrays
Using a fixed concentration of N-TSHR-mAbs (1 μg/mL) in fresh modified DMEM basal medium, cells were stimulated for 24 hours at 37 °C in the incubator. Cells were then washed twice with ice-cold PBS (pH 7.2) without calcium and magnesium (Mediatech Inc), scraped into cold lysis buffer provided by the Kinexus Co containing different phosphatase and protease inhibitor cocktails (Complete Mini; Roche Applied Science; phenylmethanesulfonyl fluoride; Sigma; and 1% Triton X-100), and sonicated and analyzed as described earlier [13]. All signals were investigated by proteomic arrays performed at the Kinexus Co. Arrays were performed with control mAb, and N-TSHR-Abs (MC1) as described [7]. A greater than 20% increase from control was considered statistically significant.
Protein Quantitation
The LI-COR In-Cell Western (ICW) assay is a quantitative immunofluorescence assay performed in microplates (optimized for 96- or 384-well format) that combines the specificity of WB with the replicability and throughput of an enzyme-linked immunosorbent assay. ICW assays are replicable and precise (LI-COR Biosciences). Infrared fluorescent dye (IRDye) subclass-specific antibodies react with the heavy (γ) chain only of the primary antibody (see Table 1). LI-COR reagents were used to detect the level of protein expressions in fixed and permeabilized cells according to the protocol provided with the kit. The plate was scanned using an Odyssey CLx Infrared Imaging System, and FI was measured using Image Studio Software. The sensitivity and specificity of ICW was more robust than Western immunoblot as observed earlier [5, 6, 8, 10].
Cytokine and Chemokine Arrays
Following a 24-hour treatment of fibroblasts with N-TSHR-mAbs, the levels of cytokines and chemokines in cell lysates were measured using the Raybiotech array service (Raybiotech Life Inc). On the Mouse Inflammation Array G1 kit, chemokines and cytokines were measured. This semi-quantitative sandwich-based test on glass slides served as the basis for this assay. Duplicate or quadruplicate slides were scanned using FI. By using cytokine arrays, we were able to compare various cell treatments with precise and effective multiplex cytokine quantification in an array format.
Statistical Analysis
Two-tailed paired t tests were used to evaluate the statistical significance of differences in means for continuous variables. P less than or equal to .05 was used to determine statistical significance. Data are means ± SEM.
Results
Thyrotropin Receptor Characterization and Induction of Reactive Oxygen Species
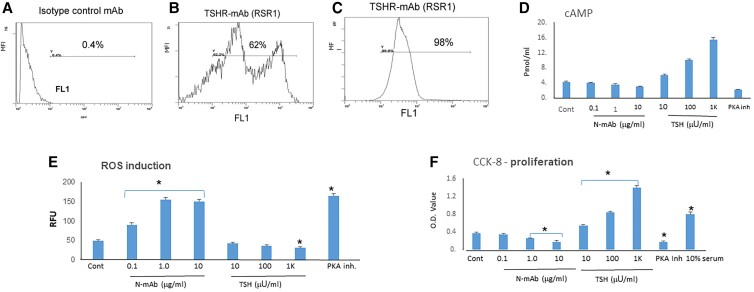
We have previously shown that both mouse and human fibroblasts respond in a similar way to TSHR Ab–induced stress [6]. To again confirm expression of the TSHR on mouse 3T3 fibroblasts, the primary model in the present studies, we performed flow cytometry using a TSHR-specific mAb (RSR1). Fig. 1B shows that 62% cells were positive for TSHR using 3T3 fibroblasts compared to the 98% positive thyroid cells (Fig. 1C). As expected, the N-TSHR-mAbs were unable to induce cAMP (in doses of 0.1-10 μg/mL) (Fig. 1D). In contrast, TSH activated cAMP generation in a concentration-dependent manner (in doses of 10-1000 μU/mL), confirming functional TSHRs on these fibroblasts. As in our earlier observations in rat thyrocytes (FRTL-5) [10], the N-TSHR-mAbs induced ROS in fibroblasts in a dose-dependent manner, while TSH suppressed ROS induction (Fig. 1E). As a control, we used a small-molecule PKA inhibitor (H89) [14], which caused an elevated level of ROS production in the fibroblasts. cAMP/PKA signaling is the master regulator of cell proliferation and growth and was not induced by N-TSHR, which appeared to reduce growth, while TSH induced a 3- to 4-fold increase (Fig. 1F). These data indicated that fibroblasts expressed functional TSHRs, which in the presence of neutral antibodies, generated statistically significant levels of ROS and suppressed proliferation as seen previously in thyroid cells.
Figure 1.
Fibroblasts respond to thyrotropin (TSH) and neutral TSH receptor antibodies (N-TSHR-mAbs) in the same way as thyroid cells. A, Fluorescence-activated cell sorter analysis with control mAbs had 0.4% binding. B, TSHR-mAb (RSR1) showed 2 cell populations totaling 62% expressing the TSHR. C, Rat thyrocytes (FRTL-5) demonstrated 98% TSHR expression. D, N-TSHR-mAbs failed to induce cyclic adenosine monophosphate (cAMP) production in fibroblasts while TSH enhanced cAMP generation and protein kinase A (PKA) inhibitor-H89 showed suppression of cAMP. E, N-TSHR-mAbs and the PKA inhibitor induced reactive oxygen species (ROS) generation while TSH suppressed ROS. F, TSH induced fibroblast proliferation while the N-TSHR-mAbs and PKA inhibitor appeared to suppress fibroblast proliferation while TSH enhanced proliferation. *P less than .05.
Reactive Oxygen Species Is Compartmentalized in Mitochondria and Endoplasmic Reticulum
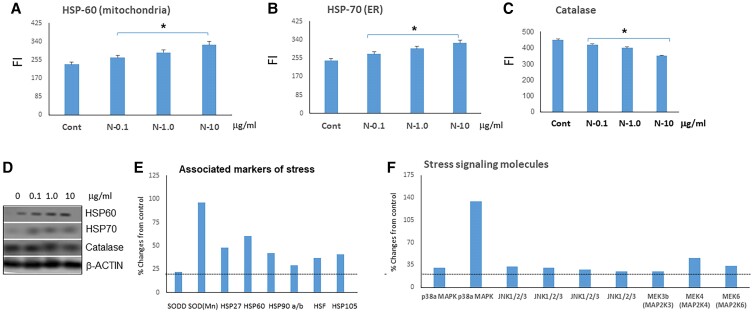
Since the N-TSHR-mAbs were unable to activate cAMP/PKA/PI3K/Akt signaling cascades, we hypothesized that multiple organelles may be subject to increased cytoplasmic stressors. Using protein markers, we measured stress levels in the ER and mitochondria that expressed heat shock proteins (HSP) when undergoing stress. HSP60 and HSP70 are specific to mitochondria and the ER, respectively, and N-TSHR-mAbs induced both mitochondria and ER stresses. Compared to control-mAb treatment, we observed a statistically significant elevation of HSP60 and HSP70 (Fig. 2A and 2B). We also looked for an antioxidant response element, particularly catalase, and observed that N-TSHR-mAbs reduced catalase levels in a dose-dependent manner by 20% to 25% (Fig. 2C). A proteomic array allowed us to examine multiple stress markers and showed especially increased levels of superoxide dismutases (SODs) (a 5-fold increase in SODs) as well as SODD, HSP27, HSP60, HSPs90, HSF, and HSP105. The array also revealed increased levels of stress-related signaling molecules (Figs. 2D-2F), including p38α-MAPK (mitogen-activated protein kinase), Jnk1/2/3 (Janus kinases), MAP2K3, MAP2K4 and MAP2K6.
Figure 2.
Neutral thyrotropin receptor antibodies (N-TSHR-mAbs) induced fibroblast cell stress as seen in thyroid cells. In fibroblasts, N-TSHR-mAbs activated both A, mitochondrial (HSP60) and B, endoplasmic reticulum (ER) stress (HSP70) in a dose-dependent manner, while C, the antibody suppressed the antioxidant protein catalase. D, These findings were also confirmed by Western blots. E, Proteomic array data also revealed ER and mitochondrial stresses. F, Similarly, N-TSHR-mAbs also induced stress-related signaling molecules.
Neutral Thyrotropin Receptor Antibodies Appear to Induce Multiple Receptor Phosphorylations
N-TSHR-mAbs not only induced panspecific proteins but also activated phosphorylation of threonine and tyrosine residues (Supplementary Fig. S1A) [15]. Decisions leading to fibroblast activation or suppression by a TSHR-mAb depend not just on receptor recognition by the TSHR but most likely also on the response of related receptors that fine-tune the cells to vital cues in their environment. N-TSHR-mAbs increased phosphorylation of the EGFR, IGF1R, and IR compared to control mAbs. N-TSHR-mAbs also appeared to induce multiple immune-related proteins, including NFκB, SOCS, Stat, Syk, Zap 70, and Lck (Supplementary Fig. S1B) [15].
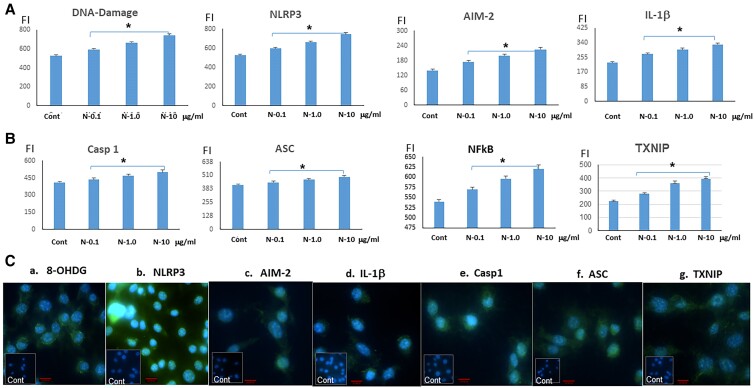
Induction of Mitochondrial DNA Damage and Inflammasome Activation
The majority of adenosine triphosphate (ATP) is produced in mitochondria through the process of oxidative phosphorylation (OXPHOS), and in excess will result in mitochondrial stress and ROS generation and the induction of mitochondrial DNA damage [16]. To examine DNA damage induced by the N-TSHR mAbs, we measured a variety of associated response elements (Fig. 3). Interleukin-1 (IL-1) secretion, a strong proinflammatory mediator, is known to be closely regulated by the “inflammasome,” which refers to a group of differing cytosolic complexes. The nucleotide-binding domain and leucine-rich repeat pyrin domain containing 3 (NLRP3) family members forms cytosolic oligomers with apoptosis-associated speck-like protein (ASC), leading to autocatalytic activation of caspase-1. In turn, caspase-1 cleaves pro–IL-1 to create mature IL-1. Pro–IL-1 and NLRP3 expression are induced by the initial signal (signal 1), which is frequently nuclear factor κB (NF-κB) activation. NF-κB is involved in the transcriptional activation of proinflammatory cytokines and other mediators, while inflammasomes contribute to the processing and release of active forms of these cytokines. The second signal might be any of a number of unrelated substances, such as extracellular ATP, crystals, protein aggregates, or particulate debris. We found that NLRP3, AIM-2, caspase 1, ASC, IL-1β, and NF-κB were all increased by the N-TSHR-mAbs dose-dependently (see Fig. 3), confirming inflammasome activation. Furthermore, thioredoxin-interacting protein (TXNIP) is a multifunctional protein that plays an important role in cellular redox regulation and apoptosis. One of the mechanisms by which TXNIP induces oxidative stress (OS) and free radicals involves its interaction with thioredoxin, a key regulator of cellular redox balance. We found that TXNIP was activated by N-TSHR-mAbs either directly by the mitochondrial ROS or by the activation of the inflammatory reactome.
Figure 3.
DNA damage induction and subsequent activation of the inflammasome. A, Neutral thyrotropin receptor antibodies (N-TSHR-mAbs) induced DNA damage along with NLRP3, AIM-2, and interleukin (IL)-1β. B, The antibody also induced caspase 1, ASC, NFκB, and TXNIP. C(a-g), Immunocytochemistry also revealed similar effects including DNA damage protein (8-OHDG), NLRP3, AIM-2, Casp1, Asc, and TXNIP. Red bar = 100 μm; *P less than .05.
Neutral Thyrotropin Receptor Antibodies Induce Suppression of Autophagy
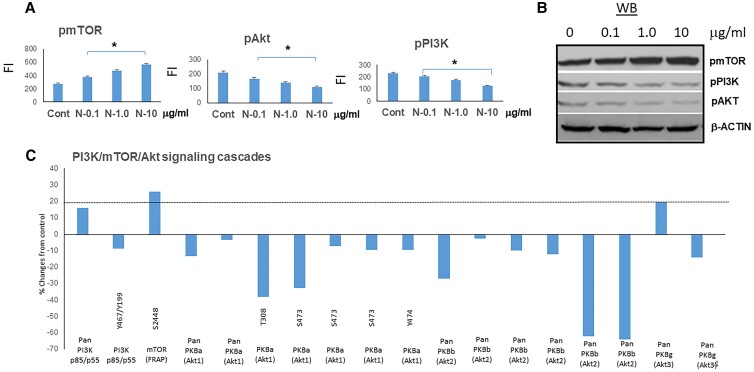
In normal and pathological settings, phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathways are essential for cell growth and survival. N-TSHR-mAbs induced mTOR activation and showed PI3/Akt suppression (Fig. 4A). This indicated suppression of autophagy leading to cell death. These findings were paralleled by the proteomic array data (Fig. 4B). Autophagy markers such as Beclin 1, LC3A, LC3B, ULK, and p62 were all reduced in a dose-dependent manner (Supplementary Fig. S2) [15] by N-TSHR-mAbs compared to control mAb treatment. Casp 11, a noncanonical pathway of autophagy, was also reduced as was pink, a marker of mitophagy. TFAM1, a marker of mitochondrial biogenesis, was similarly suppressed. These results indicated that autophagy, a cell survival signaling system, was suppressed in these fibroblasts by N-TSHR-mAbs.
Figure 4.
Activation of pmTOR and suppression of PI3K/Akt signaling by neutral thyrotropin receptor antibodies (N-TSHR-mAbs). A, In-Cell Western data of pmTOR/pPI3K/pAKT protein cascades induced and suppressed by N-TSHR-mAbs. B, Similar data were illustrated by Western blot (WB). C, Further confirmation was found with pmTOR/pPI3K/pAKT signaling kinases shown by proteomic array.
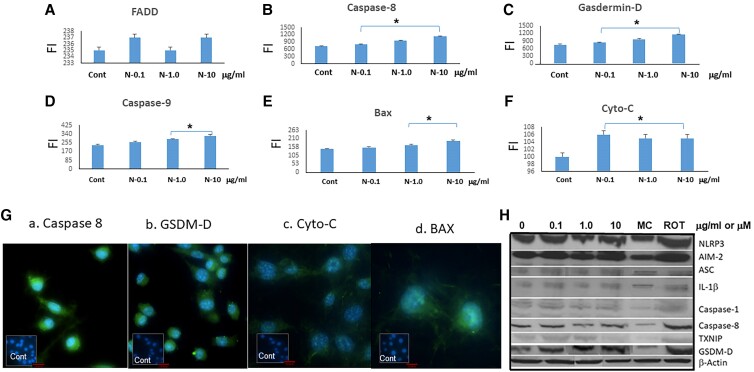
Neutral Thyrotropin Receptor Antibodies Induce Fibroblast Cell Death by Pyroptosis
Immunogenic cell death refers to cell death that generates adaptive immunity against endogenous or exogenous antigens carried by the dying cells. Pyroptosis is a type of cell death culminating in the loss of plasma membrane integrity and induced by activation of inflammasome sensors. These include the Nod-like receptor family, the DNA receptor absent in melanoma 2, and the Pyrin receptor. Inflammasome sensors detect a variety of pathogen-associated molecular pattern molecules and damage-associated molecular pattern molecules released by infecting microbes or through dysregulated cellular pathways. Gasdermin D (GSDMD), which is cleaved by caspase-8, leads its oligomerization and pore formation leading to pyroptosis [17]. Since inflammasome formation and caspase activation could be mediators for pyroptosis, we measured GSDMD expression after treating the fibroblasts with N-TSHR-mAbs. As seen in Fig. 5, GSDMD was increased by the antibody indicating the induction of rapid cell death by cell membrane rupture and resembling pyroptosis. In support of this mechanism, we found caspase 8, caspase 9, and Bax levels were all increased in the treated fibroblasts (see Fig. 5). These data support the mechanism of inflammasome-mediated induction of GSDMD in these fibroblasts leading to cell death by pyroptosis [18].
Figure 5.
Pyroptosis induction. A to F, Neutral thyrotropin receptor antibodies (N-TSHR-mAbs) induced B, caspase 8; C, gasdermin-D (GSDM-D); D, caspase-9; E, Bax; and F, cytochrome-C but not A, FADD, indicative of the onset of pyroptosis. *P less than .05. G, Immunochemistry revealed similar data. Red bar = 100 μm. H, Western blotting added further confirmation to these observations. MC (MCC950 = 5μM, NLRP3 inhibitor) and rotenone (Rot = 10μM) were used to suppress NLRP3 or to induce free radicals, respectively.
Neutral Thyrotropin Receptor Antibodies Activate Selected Cytokines and Chemokines
Using an antibody array for cytokines and chemokines, we found that the influence of the N-TSHR-mAbs was limited to a select number of cytokines and chemokines. Among the cytokines, we again found that IL-1β and IL-6 were significantly elevated and chemokines i-TAC and CxCL5 were also increased (Supplementary Fig. S3) [15]. Such changes would attract activated T cells into the tissues, including the retro-orbital eye tissues, but there was marked inhibition of SDF-1 and CXCL12, IL-3, and TNFR-1. These findings indicated that the N-TSHR-mAbs were able to modulate inflammatory responses involving chemokines and cytokines but to a variable extent.
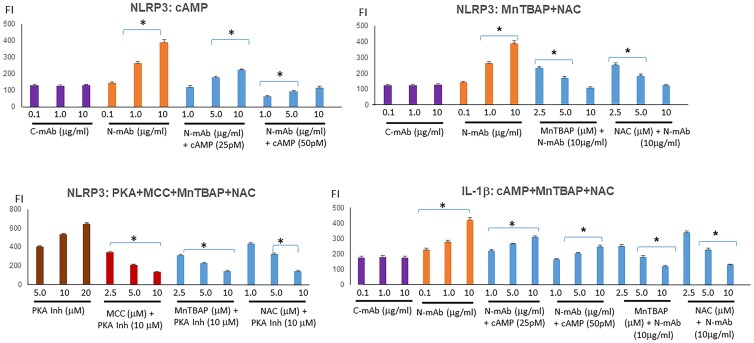
Cyclic Adenosine Monophosphate/Protein Kinase A Are Negative Regulators of Inflammasome Formation
Treating fibroblasts with cAMP and ROS inhibitors (NAC, n-acetyl-l-cystine, or MnTBAP, a SOD mimetic) directly inhibited the production of inflammasomes as demonstrated by the suppression of ROS, NLRP3, and IL-1β (Fig. 6). We confirmed that cAMP/PKA functioned as a negative regulator of inflammasome activation since a PKA inhibitor enhanced ROS (see Fig. 1E) and also induced inflammasome production with pyroptosis (not shown).
Figure 6.
Inflammasome activator induces and antioxidants/inhibitors suppress. Neutral thyrotropin receptor antibodies (N-TSHR-mAbs) induced NLRP3 and interleukin (IL)-1β production. Similar to MnTBAP and NAC (n-acetyl-l-cystine), cyclic adenosine monophosphate (cAMP) also reduced free radicals and inhibited inflammasomes including IL-1β. *P less than .05.
Discussion
Graves disease (GD) is a systemic autoimmune disorder mostly affecting the thyroid, the orbits, and skin. The relationship between these tissues is thought to depend on their expression of the TSHR, which is the main antigen for GD. Why the retro-orbital fibroblasts appear to express a greater concentration of TSHRs compared to other fibroblasts is unclear but likely secondary to a proinflammatory environment. This is important to put in perspective because stimulating TSHR-Abs are present in GD and are considered the major cause of the disorder [19, 20], and the higher the level of such antibodies the greater the chance of developing TED [21, 22]. In contrast, at least 2 other types of TSHR-Abs can be found in these patients, suggesting the presence of a variable reactome in GD patients. The blocking TSHR-Ab inhibits the action of TSH on the TSHR [23], and the third type of TSHR-Ab, often referred to as “neutral” (N-TSHR-Abs) or “hinge” region antibodies, do not interfere with TSH action but can induce stress and ROS accumulation in thyroid cells leading to cell death [5]. The role of blocking and neutral TSHR-Abs in TED remains unclear.
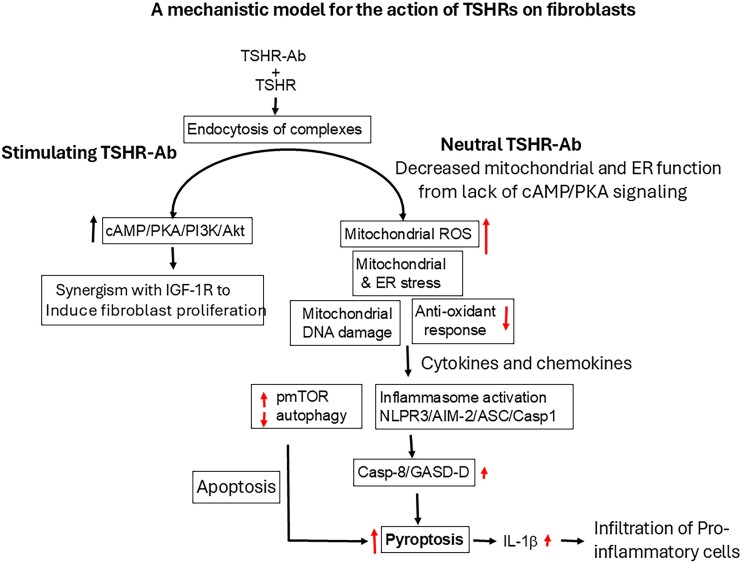
To help understand what role the neutral autoantibodies play in their interaction with the TSHRs on fibroblasts in TED, we undertook a series of tests to define fibroblast TSHR expression and their functional characteristics including their induction of stress levels and ROS. This series of interactions are summarized in Fig. 7. N-TSHR-Abs are unable to activate cAMP/PKA but are aggressive activators of ROS. OS harms a number of cellular organelles, including the mitochondria, nucleus, ER, and lysosomes [5]. When there is an imbalance between the production of free radicals and the cells capacity to eliminate them, OS results. An overabundance of peroxynitrite and hydroxyl radicals can lead to lipid peroxidation, which harms lipoproteins and cell membranes. In addition, molecular chaperones known as HSPs are generated in response to OS [24] that attempt to help in the folding of proteins to reduce stress. Our studies revealed that HSP60 and HSP70 were increased by N-TSHR-mAb treatment in fibroblasts similar to our observations in thyrocytes [5, 8]. These results indicated that the N-TSHR-mAbs created ROS-induced stress seen in different cellular compartments, including the ER and mitochondria. The increased ROS also suppressed antioxidant responses as indicated by decreasing catalase expression. Furthermore, many other stress-signaling markers (p38α, Jnk1/2/3, MAPK2K3, MAPK2K4, and MAP2K6) were all increased by N-TSHR-mAbs. These findings confirm that, in fibroblasts, ROS activation was induced by N-TSHR-Abs along with downstream signaling cascades as seen in thyroid cells and suggests they may play an important role in the retro-orbital inflammatory reaction seen in TED.
Figure 7.
A model of neutral thyrotropin receptor antibody (N-TSHR-mAb) action on fibroblasts. After endocytosis of N-TSHR-mAb/TSHR complexes into the cytoplasm of cells, there is no cyclic adenosine monophosphate (cAMP) generation but rather reactive oxygen species induction in the mitochondria due to the lack of cAMP/PKA/PI3K/Akt signaling molecules. Both mitochondria and the endoplasmic reticulum undergo stress and have compromised function leading to mitochondrial DNA damage together and failure of autophagy to protect the cells. These effects cause activation of inflammasomes via NLRP3/AIM-2/Casp1/ASC signaling cascades leading to the activation of Casp-8 and gasdermin-D (GSDM-D), ultimately causing cell death by pyroptosis and interleukin (IL)-1β secretion.
Phosphorylation is usually indicative of active G protein–coupled receptors such as the TSHR and is a crucial step in defining the signaling characteristics of this receptor superfamily. Multiple phosphorylation of receptors can occur at various intracellular locations [25]. We found that N-TSHR-mAbs induced phosphorylation of the IGF-1R more than other receptors and detected activation of many immune signaling molecules, especially IL-1β, IL-6, NFκB, Stat 1A, STAT 3, STAT 5B, Zap70, Syk, Lck, and IRAK4. When we looked into several key regulatory signaling cascades induced by the N-TSHR-mAbs, we also found considerable effects on well-defined signaling pathways, including the PKC, Ras, and transforming growth factor β (TGF-β) pathways (see Supplementary Fig. S4) [15]. Protein kinase C, also known as PKC, is a family of protein kinase enzymes that phosphorylate the hydroxyl groups of serine and threonine amino acid residues. Many biological processes, including the control of gene expression and cell division, are regulated by such PKC-mediated phosphorylation [26]. Not all PKC isoenzymes were activated and some were downregulated. Many Ras signaling molecules were also found to be increased, and the Ras/Raf/MAPK pathway is a well-studied signal transduction route that activates genes necessary for cell growth, division, and differentiation. TGF-β signaling molecules, which are also associated with cell growth and differentiation and cellular homeostasis, were also increased. As TGF-β signaling complexes of R-SMAD/coSMAD form and build up in the nucleus, they function as transcription factors and take a role in controlling the expression of target genes [27]. Clearly our results indicate that activation of these different signaling cascades may represent overactivation as a cell-survival strategy to try to prevent cell death.
We also found activation of additional signaling molecules, including NF-κB and TXNIP with downregulation of the antioxidant catalase. TXNIP can overwhelm antioxidant defenses and induce OS by inhibiting thioredoxin, adding to the accumulation of ROS. Increased OS can activate NF-κB, which in turn induces the expression of inflammatory genes. In contrast, catalase normally mitigates OS by breaking down hydrogen peroxide, potentially reducing the activation of NF-κB and the subsequent inflammatory response. NF-κB can also regulate the expression of antioxidant genes, including those encoding catalase, providing a feedback mechanism to control OS and inflammation.
We found induction of the inflammasome by N-TSHR-Ab. The inflammasome is an important part of the innate immune system that promotes the development of proinflammatory cytokine responses through proteolytic processing [28, 29]. We found that the N-TSHR-mAbs induced NLRP3, AIM 2, caspase 1, ASC, and IL-1β. In the canonical pathway, pathogen-associated molecular pattern molecules and damage-associated molecular pattern molecules receive intracellular signaling molecule stimulation and assemble with procaspase-1 and ASC to form inflammasomes and active caspase-1 [30]. Cleaved-caspase-1 itself then cleaves GSDMD and pro–IL-1β/18 and perforates the cell membrane by forming nonselective pores, further causing water influx, lysis, and cell death called pyroptosis. In addition, IL-1β and IL-18 are secreted from the pores formed by GSDMD. Our observations identified that N-TSHR-mAbs are capable of activating caspase 8 associated with GSDMD overexpression and leading to cell death by pyroptosis. The pores then release inflammatory factors, such as Il-1β, HMGB1, LDH, and ATP, which act as alarm signals to activate and recruit immune cells and mediate the immune response [29].
When a cell is starved or under stressful circumstances, it can survive by self-degrading through a process called autophagy. The mTOR is essential for regulating the ratio of cellular anabolism to catabolism and regulates many important processes, such as autophagy. The mTOR pathway integrates inputs from at least 5 primary intracellular and extracellular stimuli, including growth hormones, stress, energy status, oxygen, and amino acids [31]. The PI3K/protein kinase B (AKT) signaling pathway is also involved in the regulation of multiple cellular physiological processes by activating downstream effector molecules, which serve an important role in growth and proliferation. Our findings indicated that suppression of PI3K/AKT was accompanied by increased mTOR activity. Furthermore, autophagy markers were found to be suppressed by N-TSHR-mAbs and suppressed autophagy leads to cell death. To complicate matters even more, ROS appear to also trigger signaling pathways that actually activate autophagy to form a negative feedback loop that may suppress ROS itself [32]. We again showed that the cAMP/PKA signaling cascade also inhibited ROS when using a cAMP analogue that decreased IL-1β and the inflammasome. It has been reported [33] that the NLRP3 protein is ubiquitinated as a result of PKA phosphorylation of the inflammasome. Ubiquitination directs proteins to the proteasome, where they are broken down and recycled [34]. These findings with cAMP/PKA inhibitor made it abundantly evident that the suppression of the inflammasome was another negative feedback loop.
In conclusion, we found evidence that the fibroblast responds to a TSHR-Ab in much the same way as a thyroid cell [5, 6], with the potential for cell death resulting in aggravation of a local immune response. Once the neutral antibody binds with the TSHR forming an antigen-antibody complex, these complexes are internalized without any TSH-induced positive signaling, which then results in intracellular stress and redox activation, which damages mitochondrial DNA leading to the activation of the inflammasome complex (NLRP3/AIM-2/Casp1/Asc/IL-1β) and subsequent activation of caspase-8 and GSDMD, indicating pyroptosis induction. These signaling cascades induce secretion of IL-1β, inviting inflammatory infiltrates. This mechanistic model proposes that these TSHR-Abs initiate an inflammatory response in the retro-orbital tissues by activating inflammasomes within orbital fibroblasts. This leads to the release of proinflammatory cytokines, perpetuating chronic inflammation and driving the pathogenesis of TED, in which TSHR overexpression and high levels of a variety of TSHR-Abs are likely to be present. These molecular changes shown by the fibroblast help define the local proinflammatory response initiated by the TSHR autoantibody reactome.
Acknowledgments
We thank Drs Mihaly Mezai and Rauf Latif for their help and discussions during these studies.
Abbreviations
- AKT
protein kinase B
- ASC
apoptosis-associated speck-like protein
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- CCK-8
cholecystokinin-octapeptide
- ER
endoplasmic reticulum
- GD
Graves disease
- GSDMD
gasdermin D
- HSP
heat shock protein
- ICC
immunocytochemistry
- ICW
In-Cell Western
- IGF-1R
insulin-like growth factor-1 receptor
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- mROS
mitochondrial reactive oxygen species
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor κB
- NLR
leucine-rich repeat
- NLRP3
nucleotide-binding domain and leucine-rich repeat pyrin domain containing 3
- N-TSHR-mAbs
neutral thyrotropin receptor antibodies
- OS
oxidative stress
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol-3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TED
thyroid eye disease
- TGF-β
transforming growth factor β
- tROS
total reactive oxygen species
- TSH
thyrotropin
- TSHR
thyrotropin receptor
- TSHR-Abs
thyrotropin receptor antibodies
- TXNIP
thioredoxin-interacting protein
- UPR
unfolded protein response
- WB
Western blot
Contributor Information
Syed Morshed, Thyroid Research Unit, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA; James J. Peters VA Medical Center, Bronx, NY 10468, USA.
Maryam Mansoori, Thyroid Research Unit, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA; James J. Peters VA Medical Center, Bronx, NY 10468, USA.
Terry F Davies, Email: terry.davies@mssm.edu, Thyroid Research Unit, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA; James J. Peters VA Medical Center, Bronx, NY 10468, USA.
Funding
This work was supported in part by a Senior Clinical Investigator Award to TFD from the Veterans Administration - 2101BX000800-13.
Author Contributions
S.M. performed the experiments with the help of M.M., planned the figures, and helped interpret the data. T.F.D. designed and supervised the studies, interpreted the data, and wrote the manuscript.
Disclosures
No competing financial interests exist.
Data Availability
Some or all data sets generated during and/or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. J Clin Endocrinol Metab. 2015;100(3):1071‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010;20(1):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341‐352. [DOI] [PubMed] [Google Scholar]
- 5. Morshed S, Latif R, Davies TF. Rescue of thyroid cells from antibody induced cell death via induction of autophagy. J Autoimmun. 2022;126:102746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morshed SA, Ma R, Latif R, Davies TF. Mechanisms in graves eye disease: apoptosis as the end point of insulin-like growth factor 1 receptor inhibition. Thyroid. 2022;32(4):429‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morshed SA, Ando T, Latif R, Davies TF. Neutral antibodies to the TSH receptor are present in Graves’ disease and regulate selective signaling cascades. Endocrinology. 2010;151(11):5537‐5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morshed S, Latif R, Davies TF. Signal responses to neutral TSH receptor antibody—a cycle of damage in the pathophysiology of Graves’ disease. J Autoimmun. 2023;136:103012. [DOI] [PubMed] [Google Scholar]
- 9. Morshed SA, Ma RS, Latif R, Davies TF. Cleavage region thyrotropin receptor antibodies influence thyroid cell survival in vivo. Thyroid. 2019;29(7):993‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morshed SA, Ma R, Latif R, Davies TF. How one TSH receptor antibody induces thyrocyte proliferation while another induces apoptosis. J Autoimmun. 2013;47:17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol. 2015;11(3):171‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neag EJ, Smith TJ. 2021 update on thyroid-associated ophthalmopathy. J Endocrinol Invest. 2022;45(2):235‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150(1):519‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giacoletti G, Price T, Hoelz LVB, et al. A selective adenylyl cyclase 1 inhibitor relieves pain without causing tolerance. Front Pharmacol. 2022;13:935588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies T. Supplementary Figures. 2024. https://figshare.com/articles/figure/_/27105973
- 16. Próchnicki T, Vasconcelos MB, Robinson KS, et al. Mitochondrial damage activates the NLRP10 inflammasome. Nat Immunol. 2023;24(4):595‐603. [DOI] [PubMed] [Google Scholar]
- 17. Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888‐E10E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diana T, Ponto KA, Kahaly GJ. Thyrotropin receptor antibodies and Graves’ orbitopathy. J Endocrinol Invest. 2021;44(4):703‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91(9):3464‐3470. [DOI] [PubMed] [Google Scholar]
- 21. Nicolì F, Lanzolla G, Mantuano M, et al. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves’ orbitopathy. J Endocrinol Invest. 2021;44(3):581‐585. [DOI] [PubMed] [Google Scholar]
- 22. George A, Diana T, Längericht J, Kahaly GJ. Stimulatory thyrotropin receptor antibodies are a biomarker for Graves’ orbitopathy. Front Endocrinol (Lausanne). 2020;11:629925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diana T, Olivo PD, Kahaly GJ. Thyrotropin receptor blocking antibodies. Horm Metab Res. 2018;50(12):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Yu M, Cui J, Du Y, Teng X, Zhang Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-kappaB pathway in excess manganese-treated chicken livers. Ecotoxicol Environ Saf. 2021;226:112833. [DOI] [PubMed] [Google Scholar]
- 25. Patwardhan P, Miller WT. Processive phosphorylation: mechanism and biological importance. Cell Signal. 2007;19(11):2218‐2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scerri J, Scerri C, Schäfer-Ruoff F, Fink S, Templin M, Grech G. PKC-mediated phosphorylation and activation of the MEK/ERK pathway as a mechanism of acquired trastuzumab resistance in HER2-positive breast cancer. Front Endocrinol (Lausanne). 2022;13:1010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114(Pt 24):4359‐4369. [DOI] [PubMed] [Google Scholar]
- 28. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 29. Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165(4):792‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnett KC, Li S, Liang K, Ting JP. A 360 degrees view of the inflammasome: mechanisms of activation, cell death, and diseases. Cell. 2023;186(11):2288‐2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tedesco B, Vendredy L, Timmerman V, Poletti A. The chaperone-assisted selective autophagy complex dynamics and dysfunctions. Autophagy. 2023;19(6):1619‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35(5):615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):944. [DOI] [PubMed] [Google Scholar]
- 34. Maxwell BA, Gwon Y, Mishra A, et al. Ubiquitination is essential for recovery of cellular activities after heat shock. Science. 2021;372(6549):eabc3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davies T. Supplementary Figures. 2024. https://figshare.com/articles/figure/_/27105973
Data Availability Statement
Some or all data sets generated during and/or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.