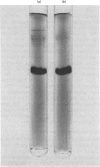
Abstract
Spermidine synthase (EC 2.5.1.16) was purified to apparent homogeneity (about 11 000-fold) from bovine brain by affinity chromatography, with S-adenosyl-(5')-3-thiopropylamine linked to Sepharose as the adsorbent. The enzyme preparation was free from S-adenosylmethionine decarboxylase (EC 4.1.1.50) and spermine synthase (EC 2.5.1.22) activities. The native enzyme had an apparent Mr of 70 000, was composed of two subunits of equal size, and had an isoelectric point at pH 5.22. The apparent Km values for putrescine and decarboxylated adenosylmethionine [S-adenosyl-(5')-3-methylthiopropylamine] were 40 microM and 0.3 microM respectively. Cadaverine and 1,6-diaminohexane could replace putrescine as the aminopropyl acceptor, although the reaction rates were only 6% and 1% respectively of that obtained with putrescine. Ethyl, propyl and carboxymethyl analogues of decarboxy-S-adenosylmethionine could act as propylamine donors. Both the reaction products, spermidine and 5'-methylthioadenosine, were mixed-type inhibitors of the enzyme. On the basis of initial-velocity and product-inhibition studies, a ping-pong reaction mechanism for the spermidine synthase reaction was ruled out.
Full text
PDF



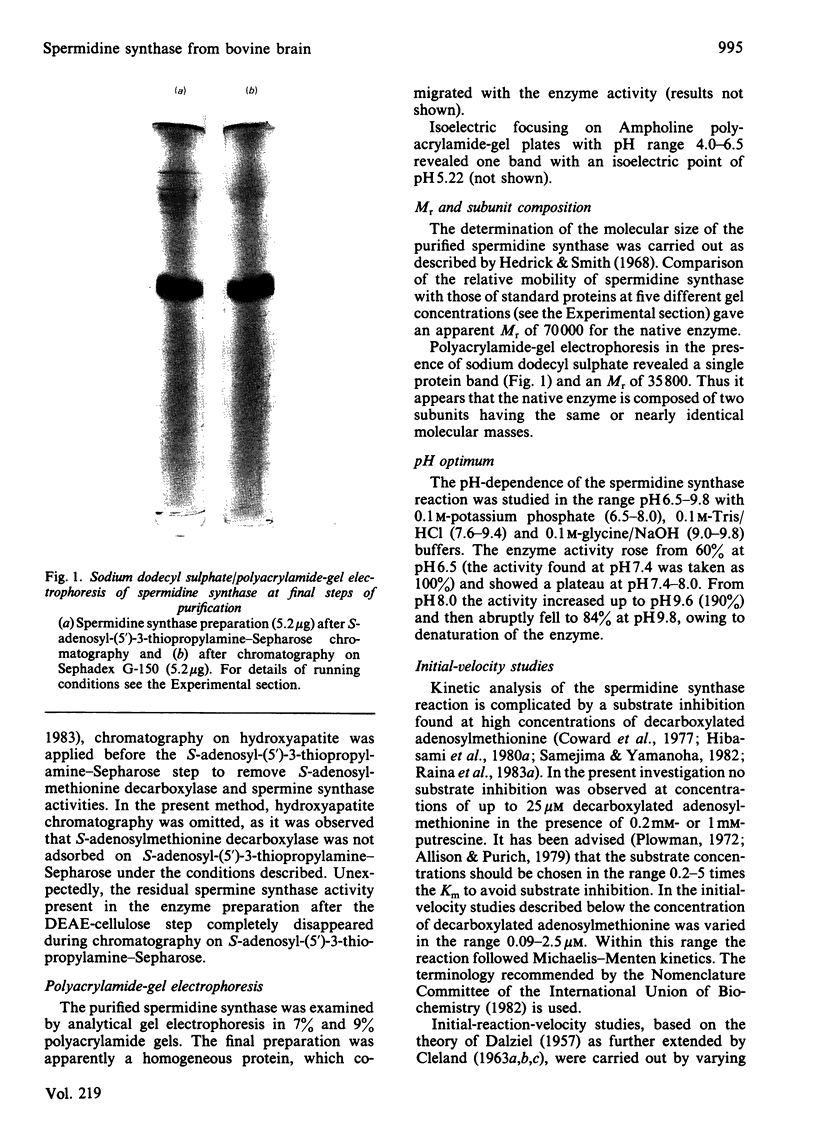



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Naji A. S., Clarke D. E. Amine oxidase activity in commercial preparations of bovine serum albumin. Life Sci. 1983 Feb 7;32(6):635–643. doi: 10.1016/0024-3205(83)90209-6. [DOI] [PubMed] [Google Scholar]
- Allison R. D., Purich D. L. Practical considerations in the design of initial velocity enzyme rate assays. Methods Enzymol. 1979;63:3–22. doi: 10.1016/0076-6879(79)63003-3. [DOI] [PubMed] [Google Scholar]
- Baici A. The specific velocity plot. A graphical method for determining inhibition parameters for both linear and hyperbolic enzyme inhibitors. Eur J Biochem. 1981 Sep;119(1):9–14. doi: 10.1111/j.1432-1033.1981.tb05570.x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Coward J. K., Motola N. C., Moyer J. D. Polyamine biosynthesis in rat prostate. Substrate and inhibitor properties of 7-deaza analogues of decarboxylated S-adenosylmethionine and 5'-methylthioadenosine. J Med Chem. 1977 Apr;20(4):500–505. doi: 10.1021/jm00214a008. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Borchardt R. T., Chen S. Y., Coward J. K., Pegg A. E. Studies of inhibition of rat spermidine synthase and spermine synthase. Biochem J. 1980 May 1;187(2):419–428. doi: 10.1042/bj1870419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Hoffman J. L., Pegg A. E. Decarboxylated S-adenosylmethionine in mammalian cells. J Biol Chem. 1980 Jul 25;255(14):6675–6678. [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Rapid and convenient method for the assay of aminopropyltransferases. Biochem J. 1978 Mar 1;169(3):709–712. doi: 10.1042/bj1690709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Schenone A., Williams-Ashman H. G. Separation of two proteins required for synthesis of spermidine from S-adenosyl-L-methionine and putrescine in rat prostate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):758–764. doi: 10.1016/0006-291x(71)90552-3. [DOI] [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Eloranta T. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermine synthase from bovine brain. Eur J Biochem. 1979 Nov;101(2):619–626. doi: 10.1111/j.1432-1033.1979.tb19756.x. [DOI] [PubMed] [Google Scholar]
- Pankaskie M. C., Abdel-Monem M. M., Raina A., Wang T., Foker J. E. Inhibitors of polyamine biosynthesis. 9. Effects of S-adenosyl-L-methionine analogues on mammalian aminopropyltransferases in vitro and polyamine biosynthesis in transformed lymphocytes. J Med Chem. 1981 May;24(5):549–553. doi: 10.1021/jm00137a014. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Purification of rat liver S-adenosyl-L-methionine decarboxylase. Biochem J. 1974 Aug;141(2):581–583. doi: 10.1042/bj1410581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Shuttleworth K., Hibasami H. Specificity of mammalian spermidine synthase and spermine synthase. Biochem J. 1981 Aug 1;197(2):315–320. doi: 10.1042/bj1970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Persson L. Decarboxylation of ornithine and lysine by ornithine decarboxylase from kidneys of testosterone treated mice. Acta Chem Scand B. 1981;35(6):451–459. doi: 10.3891/acta.chem.scand.35b-0451. [DOI] [PubMed] [Google Scholar]
- Raina A., Eloranta T., Pajula R. L. Rapid assays for putrescine aminopropyltransferase (spermidine synthase) and spermidine aminopropyltransferase (spermine synthase). Methods Enzymol. 1983;94:257–260. doi: 10.1016/s0076-6879(83)94044-2. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Raina Aarne, Hannonen Pekka. Separation of enzyme activities catalysing spermidine and spermine synthesis in rat brain. FEBS Lett. 1971 Jul 15;16(1):1–4. doi: 10.1016/0014-5793(71)80669-5. [DOI] [PubMed] [Google Scholar]
- Rudolph F. B. Product inhibition and abortive complex formation. Methods Enzymol. 1979;63:411–436. doi: 10.1016/0076-6879(79)63018-5. [DOI] [PubMed] [Google Scholar]
- Samejima K., Nakazawa Y. Action of decarboxylated S-adenosylmethionine analogs in the spermidine-synthesizing system from rat prostate. Arch Biochem Biophys. 1980 Apr 15;201(1):241–246. doi: 10.1016/0003-9861(80)90508-1. [DOI] [PubMed] [Google Scholar]
- Samejima K., Raina A., Yamanoha B., Eloranta T. Purification of putrescine aminopropyltransferase (spermidine synthase) from eukaryotic tissues. Methods Enzymol. 1983;94:270–276. doi: 10.1016/s0076-6879(83)94047-8. [DOI] [PubMed] [Google Scholar]
- Samejima K., Yamanoha B. Purification of spermidine synthase from rat ventral prostate by affinity chromatography on immobilized S-adenosyl(5')-3-thiopropylamine. Arch Biochem Biophys. 1982 Jun;216(1):213–222. doi: 10.1016/0003-9861(82)90206-5. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Seiler N., Lamberty U. Polyamines and nucleic acids in brains and hearts of newborn and adult autophagous and heterophagous vertebrates. Comp Biochem Physiol B. 1975 Nov 15;52(3):419–425. doi: 10.1016/0305-0491(75)90155-8. [DOI] [PubMed] [Google Scholar]
- Tang K. C., Pegg A. E., Coward J. K. Specific and potent inhibition of spermidine synthase by the transition-state analog, S-adenosyl-3-thio-1,8-diaminooctane. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1371–1377. doi: 10.1016/0006-291x(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zappia V., Cacciapuoti G., Pontoni G., Oliva A. Mechanism of propylamine-transfer reactions. Kinetic and inhibition studies on spermidine synthase from Escherichia coli. J Biol Chem. 1980 Aug 10;255(15):7276–7280. [PubMed] [Google Scholar]