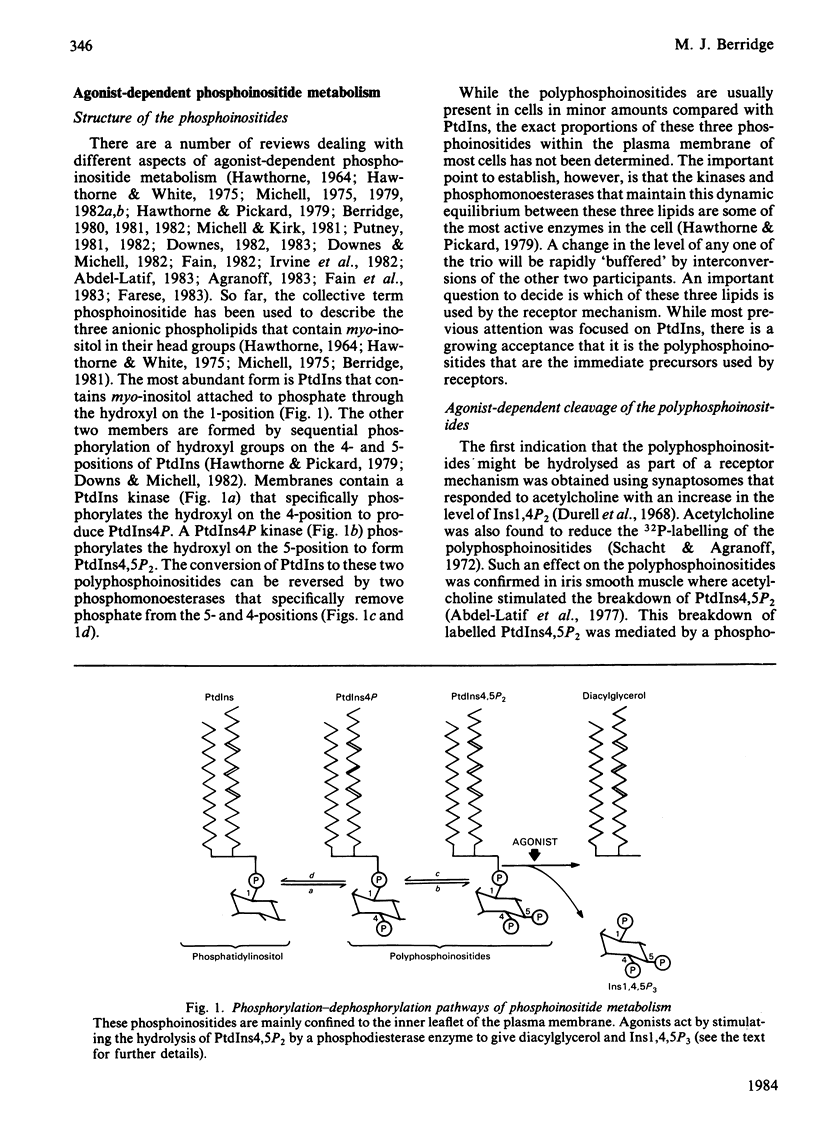
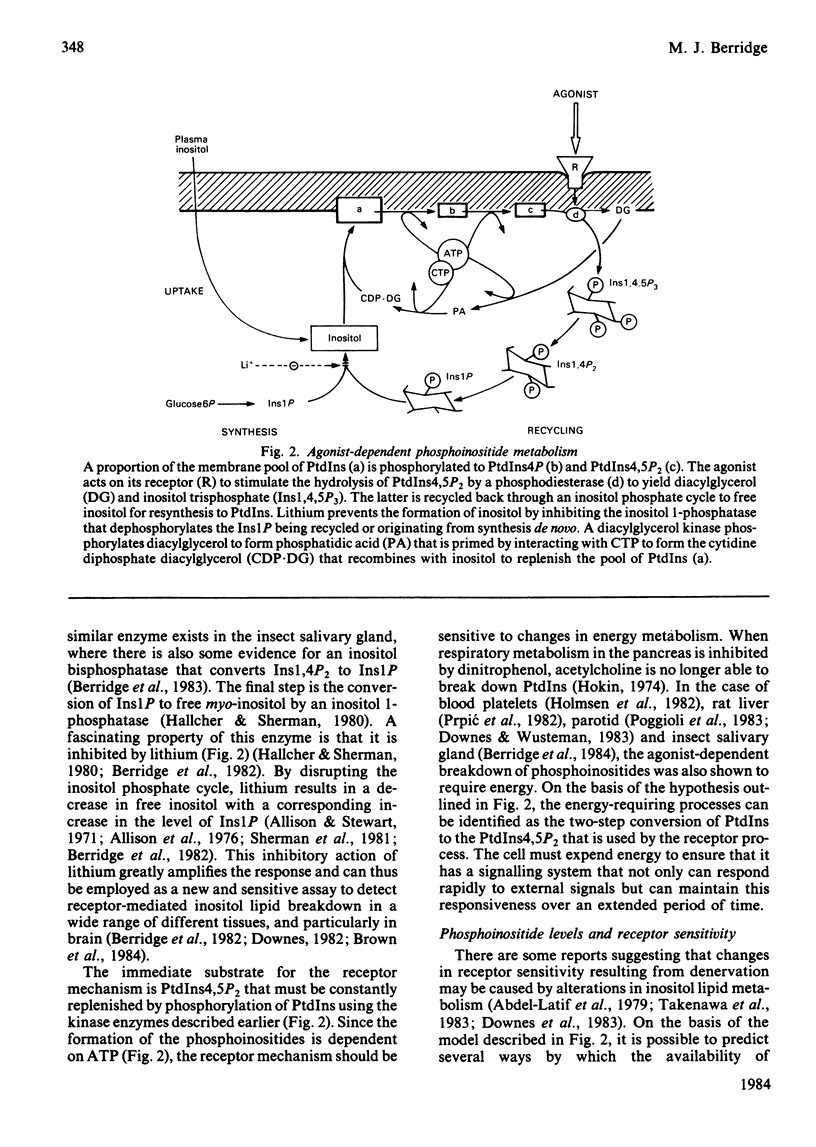
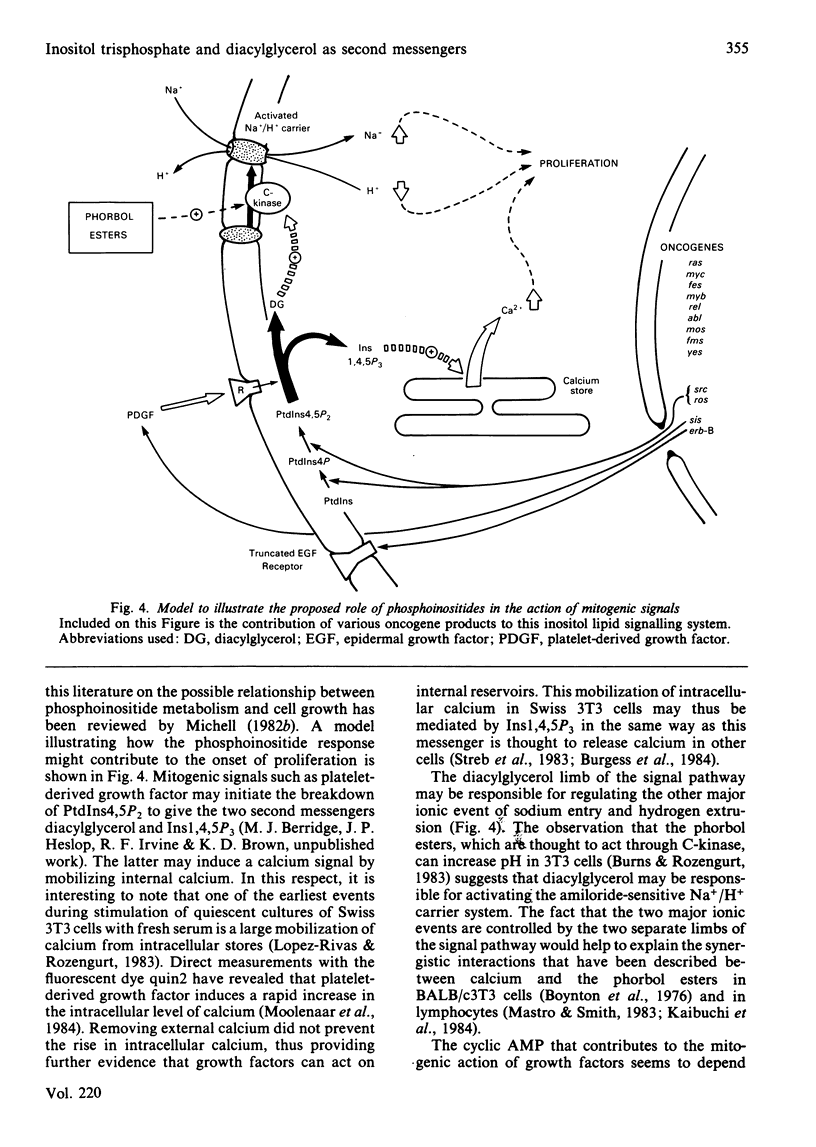
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Green K., Smith J. P. Sympathetic denervation and the triphosphoinositide effect in the iris smooth muscle: a biochemical method for the determination of alpha-adrenergic receptor denervation supersensitivity. J Neurochem. 1979 Jan;32(1):225–228. doi: 10.1111/j.1471-4159.1979.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Agranoff B. W. Biochemical mechanisms in the phosphatidylinositol effect. Life Sci. 1983 May 2;32(18):2047–2054. doi: 10.1016/0024-3205(83)90092-9. [DOI] [PubMed] [Google Scholar]
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Requirement for calcium ions in acetylcholine-stimulated phosphodiesteratic cleavage of phosphatidyl-myo-inositol 4,5-bisphosphate in rabbit iris smooth muscle. Biochem J. 1980 Dec 15;192(3):783–791. doi: 10.1042/bj1920783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. H., Blisner M. E., Holland W. H., Hipps P. P., Sherman W. R. Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem Biophys Res Commun. 1976 Jul 26;71(2):664–670. doi: 10.1016/0006-291x(76)90839-1. [DOI] [PubMed] [Google Scholar]
- Allison J. H., Stewart M. A. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971 Oct 27;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Aloyo V. J., Zwiers H., Gispen W. H. Phosphorylation of B-50 protein by calcium-activated, phospholipid-dependent protein kinase and B-50 protein kinase. J Neurochem. 1983 Sep;41(3):649–653. doi: 10.1111/j.1471-4159.1983.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Axen K. V., Schubart U. K., Blake A. D., Fleischer N. Role of Ca2+ in secretagogue-stimulated breakdown of phosphatidylinositol in rat pancreatic islets. J Clin Invest. 1983 Jul;72(1):13–21. doi: 10.1172/JCI110951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Degradation of phosphatidylinositol-4,5-bisphosphate is insensitive to CA2+ mobilization in stimulated platelets. Biochem Biophys Res Commun. 1982 Nov 16;109(1):217–222. doi: 10.1016/0006-291x(82)91587-x. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Evidence for multiple metabolic pools of phosphatidylinositol in stimulated platelets. J Biol Chem. 1982 Oct 25;257(20):11856–11859. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Billah M. M., Michell R. H. Phosphatidylinositol metabolism in rat hepatocytes stimulated by glycogenolytic hormones. Effects of angiotensin, vasopressin, adrenaline, ionophore A23187 and calcium-ion deprivation. Biochem J. 1979 Sep 15;182(3):661–668. doi: 10.1042/bj1820661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. Calcium-dependent stimulation of BALB/c 3T3 mouse cell DNA synthesis by a tumor-promoting phorbol ester (PMA). J Cell Physiol. 1976 Jan;87(1):25–32. doi: 10.1002/jcp.1040870105. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Brown S. L., Brown J. H. Muscarinic stimulation of phosphatidylinositol metabolism in atria. Mol Pharmacol. 1983 Nov;24(3):351–356. [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Serum, platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in Swiss 3T3 cells. Biochem Biophys Res Commun. 1983 Nov 15;116(3):931–938. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Calderon P., Furnelle J., Christophe J. Phosphatidylinositol turnover and calcium movement in the rat pancreas. Am J Physiol. 1980 Mar;238(3):G247–G254. doi: 10.1152/ajpgi.1980.238.3.G247. [DOI] [PubMed] [Google Scholar]
- Cassel D., Rothenberg P., Zhuang Y. X., Deuel T. F., Glaser L. Platelet-derived growth factor stimulates Na+/H+ exchange and induces cytoplasmic alkalinization in NR6 cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6224–6228. doi: 10.1073/pnas.80.20.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. Stimulus-secretion coupling in rabbit neutrophils is not mediated by phosphatidylinositol breakdown. Nature. 1980 Nov 20;288(5788):275–277. doi: 10.1038/288275a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. f-MetLeuPhe-induced phosphatidylinositol turnover in rabbit neutrophils is dependent on extracellular calcium. FEBS Lett. 1980 Jan 28;110(1):115–118. doi: 10.1016/0014-5793(80)80036-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Evidence for a role of phosphatidylinositol turnover in stimulus-secretion coupling. Studies with rat peritoneal mast cells. Biochem J. 1979 Mar 15;178(3):681–687. doi: 10.1042/bj1780681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. Phosphatidylinositol metabolism in mast cells and neutrophils. Cell Calcium. 1982 Oct;3(4-5):337–349. doi: 10.1016/0143-4160(82)90021-5. [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr, Cone C. M. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976 Apr 9;192(4235):155–158. doi: 10.1126/science.56781. [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr, Tongier M., Jr Contact inhibition of division: involvement of the electrical transmembrane potential. J Cell Physiol. 1973 Dec;82(3):373–386. doi: 10.1002/jcp.1040820307. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F. Possible role of lysosomal phospholidpases in inducing tissue prostaglandin synthesis. Adv Prostaglandin Thromboxane Res. 1978;3:47–54. [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Dibner M. D., Hanley M. R. Sympathetic denervation impairs agonist-stimulated phosphatidylinositol metabolism in rat parotid glands. Biochem J. 1983 Sep 15;214(3):865–870. doi: 10.1042/bj2140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P. Receptor-stimulated inositol phospholipid metabolism in the central nervous system. Cell Calcium. 1982 Oct;3(4-5):413–428. doi: 10.1016/0143-4160(82)90027-6. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Durell J., Sodd M. A., Friedel R. O. Acetylcholine stimulation of the phosphodiesteratic cleavage of guinea pig brain phosphoinositides. Life Sci. 1968 Apr 15;7(8):363–368. doi: 10.1016/0024-3205(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Walton J. M. Calcium ions and the control of proliferation in normal and cancer cells. Biosci Rep. 1982 Jan;2(1):15–30. doi: 10.1007/BF01142195. [DOI] [PubMed] [Google Scholar]
- Egawa K., Sacktor B., Takenawa T. Ca2+-dependent and Ca2+-independent degradation of phosphatidylinositol in rabbit vas deferens. Biochem J. 1981 Jan 15;194(1):129–136. doi: 10.1042/bj1940129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between phosphatidylinositol synthesis and recovery of 5-hydroxytryptamine-responsive Ca2+ flux in blowfly salivary glands. Biochem J. 1979 Jun 15;180(3):655–661. doi: 10.1042/bj1800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Lin S. H., Litosch I., Wallace M. Hormonal regulation of phosphatidylinositol breakdown. Life Sci. 1983 May 2;32(18):2055–2067. doi: 10.1016/0024-3205(83)90093-0. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Ca2+-dependent and Ca2+-independent mechanisms for phosphatidylinositol hydrolysis and 32P-labeling during cholinergic stimulation of the rat submaxillary gland in vitro. Arch Biochem Biophys. 1982 Nov;219(1):204–208. doi: 10.1016/0003-9861(82)90150-3. [DOI] [PubMed] [Google Scholar]
- Farese R. V. Phosphoinositide metabolism and hormone action. Endocr Rev. 1983 Winter;4(1):78–95. doi: 10.1210/edrv-4-1-78. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Hootman S. R., Heacock A. M., Ernst S. A., Agranoff B. W. Muscarinic stimulation of phospholipid turnover in dissociated avian salt gland cells. FEBS Lett. 1983 May 2;155(1):43–46. doi: 10.1016/0014-5793(83)80205-1. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hamet P., Ross A. H., Lawson J. A., Hardman J. G. Calcium-induced release from platelet membranes of fatty acids that modulate soluble guanylate cyclase. J Pharmacol Exp Ther. 1983 Jul;226(1):180–186. [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Transport of myo-inositol into endoneurial preparations of sciatic nerve from normal and streptozotocin-diabetic rats. Biochem J. 1983 Mar 15;210(3):775–781. doi: 10.1042/bj2100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE J. N. THE BIOCHEMISTRY OF THE INOSITOL LIPIDS. Vitam Horm. 1964;22:57–79. doi: 10.1016/s0083-6729(08)60336-2. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim Biophys Acta. 1955 Sep;18(1):102–110. doi: 10.1016/0006-3002(55)90013-5. [DOI] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Harris R. A., Schmidt J., Hitzemann B. A., Hitzemann R. J. Phosphatidate as a molecular link between depolarization and neurotransmitter release in the brain. Science. 1981 Jun 12;212(4500):1290–1291. doi: 10.1126/science.7233220. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Sasaki T. Rapid breakdown of phosphatidylinositol accompanied by accumulation of phosphatidic acid and diacylglycerol in rat lymphocytes stimulated by concanavalin A. J Biochem. 1982 Feb;91(2):463–468. doi: 10.1093/oxfordjournals.jbchem.a133718. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., Pickard M. R. Phospholipids in synaptic function. J Neurochem. 1979 Jan;32(1):5–14. doi: 10.1111/j.1471-4159.1979.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann R., Ristow H. J., Pachowsky H., Frank W. Phospholipid metabolism in embryonic rat fibroblasts following stimulation by a combination of the serum proteins S1 and S2. Eur J Biochem. 1974 Nov 15;49(2):317–324. doi: 10.1111/j.1432-1033.1974.tb03836.x. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Yoss N. L. Failure of phosphatidic acid to translocate Ca2+ across phosphatidylcholine membranes. Nature. 1983 Oct 13;305(5935):637–638. doi: 10.1038/305637a0. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Kaplan K. L., Dangelmaier C. A. Differential energy requirements for platelet responses. A simultaneous study of aggregation, three secretory processes, arachidonate liberation, phosphatidylinositol breakdown and phosphatidate production. Biochem J. 1982 Oct 15;208(1):9–18. doi: 10.1042/bj2080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honchar M. P., Olney J. W., Sherman W. R. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983 Apr 15;220(4594):323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. The activation of phosphatidylinositol-hydrolyzing phospholipase A2 during prostaglandin synthesis in transformed mouse BALB/3T3 cells. J Biol Chem. 1981 May 25;256(10):5215–5219. [PubMed] [Google Scholar]
- Huerta-Bahena J., García-Saínz J. A. Inositol administration restores the sensitivity of liver cells formed during liver regeneration to alpha 1-adrenergic amines, vasopressin and angiotensin II. Biochim Biophys Acta. 1983 Sep 22;763(2):125–128. doi: 10.1016/0167-4889(83)90035-6. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Harmony J. A. Phosphatidylinositol turnover in mitogen-activated lymphocytes. Suppression by low-density lipoproteins. Biochem J. 1980 Oct 15;192(1):91–98. doi: 10.1042/bj1920091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi Y., Kondo Y. Acute effect of thyrotropin on phosphatidylinositol degradation and transient accumulation of diacylglycerol in isolated thyroid follicles. Biochem Biophys Res Commun. 1980 Nov 28;97(2):759–765. doi: 10.1016/0006-291x(80)90329-0. [DOI] [PubMed] [Google Scholar]
- Imai A., Ishizuka Y., Kawai K., Nozawa Y. Evidence for coupling of phosphatidic acid formation and calcium influx in thrombin-activated human platelets. Biochem Biophys Res Commun. 1982 Sep 30;108(2):752–759. doi: 10.1016/0006-291x(82)90893-2. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Dawson R. M. The mechanism and function of phosphatidylinositol turnover [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):376–377. doi: 10.1042/bst0080376. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., van Dongen C. J., Schotman P., Wirtz K. W., Gispen W. H. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980 Aug 7;286(5773):623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Cockcroft S., Michell R. H. Stimulation of phosphatidylinositol turnover in various tissues by cholinergic and adrenergic agonists, by histamine and by caerulein. Biochem J. 1979 Sep 15;182(3):669–676. doi: 10.1042/bj1820669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. The relationship of calcium to receptor-controlled stimulation of phosphatidylinositol turnover. Effects of acetylcholine, adrenaline, calcium ions, cinchocaine and a bivalent cation ionophore on rat parotid-gland fragments. Biochem J. 1975 Jun;148(3):479–485. doi: 10.1042/bj1480479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kennerly D. A., Sullivan T. J., Sylwester P., Parker C. W. Diacylglycerol metabolism in mast cells: a potential role in membrane fusion and arachidonic acid release. J Exp Med. 1979 Oct 1;150(4):1039–1044. doi: 10.1084/jem.150.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Kirk C. J. Ligand-stimulated inositol lipid metabolism in the liver: relationship to receptor function. Cell Calcium. 1982 Oct;3(4-5):399–411. doi: 10.1016/0143-4160(82)90026-4. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. The influence of blood pressure on trans-synovial flow in the rabbit. J Physiol. 1984 Apr;349:27–42. doi: 10.1113/jphysiol.1984.sp015140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Koren R., Cass C. E., Paterson A. R. The kinetics of dissociation of the inhibitor of nucleoside transport, nitrobenzylthioinosine, from the high-affinity binding sites of cultured hamster cells. Biochem J. 1983 Nov 15;216(2):299–308. doi: 10.1042/bj2160299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G. Metabolism of inositides and the activation of platelets. Life Sci. 1983 May 2;32(18):2069–2082. doi: 10.1016/0024-3205(83)90094-2. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Wallace M. A., Fain J. N. Regulation of Ca2+-Mg2+-ATPase activity in hepatocyte plasma membranes by vasopressin and phenylephrine. Endocrinology. 1983 Dec;113(6):2268–2275. doi: 10.1210/endo-113-6-2268. [DOI] [PubMed] [Google Scholar]
- Litosch I., Lee H. S., Fain J. N. Phosphoinositide breakdown in blowfly salivary glands. Am J Physiol. 1984 Jan;246(1 Pt 1):C141–C147. doi: 10.1152/ajpcell.1984.246.1.C141. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Rozengurt E. Serum rapidly mobilizes calcium from an intracellular pool in quiescent fibroblastic cells. Biochem Biophys Res Commun. 1983 Jul 18;114(1):240–247. doi: 10.1016/0006-291x(83)91619-4. [DOI] [PubMed] [Google Scholar]
- Lopo A., Vacquier V. D. The rise and fall of intracellular pH of sea urchin eggs after fertilisation. Nature. 1977 Oct 13;269(5629):590–592. doi: 10.1038/269590a0. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Atherton R. M. Characterization of a platelet protein phosphorylated during the thrombin-induced release reaction. Biochemistry. 1979 Feb 6;18(3):544–552. doi: 10.1021/bi00570a025. [DOI] [PubMed] [Google Scholar]
- Mahadevappa V. G., Holub B. J. Degradation of different molecular species of phosphatidylinositol in thrombin-stimulated human platelets. J Biol Chem. 1983 May 10;258(9):5337–5339. [PubMed] [Google Scholar]
- Malaisse W. J., Lebrun P., Herchuelz A., Sener A., Malaisse-Lagae F. Synergistic effect of a tumor-promoting phorbol ester and a hypoglycemic sulfonylurea upon insulin release. Endocrinology. 1983 Nov;113(5):1870–1877. doi: 10.1210/endo-113-5-1870. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Press R., Altszuler N., Stewart M. A. Inositol production by the brain in normal and alloxan-diabetic dogs. Brain Res. 1971 May 21;28(3):535–539. doi: 10.1016/0006-8993(71)90061-8. [DOI] [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Mastro A. M., Smith M. C. Calcium-dependent activation of lymphocytes by ionophore, A23187, and a phorbol ester tumor promoter. J Cell Physiol. 1983 Jul;116(1):51–56. doi: 10.1002/jcp.1041160109. [DOI] [PubMed] [Google Scholar]
- Mauco G., Chap H., Douste-Blazy L. Platelet activating factor (PAF-acether) promotes an early degradation of phosphatidylinositol-4,5-biphosphate in rabbit platelets. FEBS Lett. 1983 Mar 21;153(2):361–365. doi: 10.1016/0014-5793(83)80643-7. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Pohjanpelto P., Rozengurt E. Na entry and Na-K pump activity in murine, hamster, and human cells--effect of monensin, serum, platelet extract, and viral transformation. J Cell Physiol. 1980 Apr;103(1):17–27. doi: 10.1002/jcp.1041030104. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Pozzan T., Smith G. A., Hesketh T. R. A calcium hypothesis for the control of cell growth. Biochem Soc Symp. 1980;45:1–26. [PubMed] [Google Scholar]
- Michell R. H. Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium. 1982 Oct;3(4-5):429–440. doi: 10.1016/0143-4160(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. The possible involvement of phosphatidylinositol breakdown in the mechanism of stimulus-response coupling at receptors which control cell-surface calcium gates. Adv Exp Med Biol. 1977;83:447–464. doi: 10.1007/978-1-4684-3276-3_41. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Monaco M. E. The phosphatidylinositol cycle in WRK-1 cells. Evidence for a separate, hormone-sensitive phosphatidylinositol pool. J Biol Chem. 1982 Mar 10;257(5):2137–2139. [PubMed] [Google Scholar]
- Monaco M. E., Woods D. Characterization of the hormone-sensitive phosphatidylinositol pool in WRK-1 cells. J Biol Chem. 1983 Dec 25;258(24):15125–15129. [PubMed] [Google Scholar]
- Montague W., Parkin E. N. Changes in membrane lipids of the beta-cell during insulin secretion. Horm Metab Res Suppl. 1980;Suppl 10:153–157. [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Stimulation of phosphatidic acid of calcium influx and cyclic GMP synthesis in neuroblastoma cells. J Biol Chem. 1981 Nov 10;256(21):10945–10948. [PubMed] [Google Scholar]
- Peach M. J. Molecular actions of angiotensin. Biochem Pharmacol. 1981 Oct;30(20):2745–2751. doi: 10.1016/0006-2952(81)90410-x. [DOI] [PubMed] [Google Scholar]
- Penniston J. T. Plasma membrane Ca2+-pumping ATPases. Ann N Y Acad Sci. 1982;402:296–303. doi: 10.1111/j.1749-6632.1982.tb25751.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. What is the mechanism of the calcium influx to pancreatic acinar cells evoked by secretagogues? Pflugers Arch. 1983 Jan;396(1):82–84. doi: 10.1007/BF00584703. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Weiss S. J., McKinney J. S., Putney J. W., Jr Effects of antimycin A on receptor-activated calcium mobilization and phosphoinositide metabolism in rat parotid gland. Mol Pharmacol. 1983 Jan;23(1):71–77. [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol lipids and cell stimulation in mammalian salivary gland. Cell Calcium. 1982 Oct;3(4-5):369–383. doi: 10.1016/0143-4160(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Recent hypotheses regarding the phosphatidylinositol effect. Life Sci. 1981 Sep 21;29(12):1183–1194. doi: 10.1016/0024-3205(81)90221-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Rhodes D., Prpić V., Exton J. H., Blackmore P. F. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in hepatocytes by vasopressin. J Biol Chem. 1983 Mar 10;258(5):2770–2773. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Ristow H. J., Messmer T. O., Walter S., Paul D. Stimulation of DNA synthesis any myo-inositol incorporation in mammalian cells. J Cell Physiol. 1980 May;103(2):263–269. doi: 10.1002/jcp.1041030211. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Inositol lipid metabolism in the responses of stimulated platelets. Cell Calcium. 1982 Oct;3(4-5):311–322. doi: 10.1016/0143-4160(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Roach P. J., Goldman M. Modification of glycogen synthase activity in isolated rat hepatocytes by tumor-promoting phorbol esters: evidence for differential regulation of glycogen synthase and phosphorylase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7170–7172. doi: 10.1073/pnas.80.23.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrsehneider L. R., Boutwell R. K. Phorbol esters, fatty acids and tumour promotion. Nat New Biol. 1973 Jun 13;243(128):212–213. doi: 10.1038/newbio243212a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Cyclic AMP: a growth-promoting signal for mouse 3T3 cells. Adv Cyclic Nucleotide Res. 1981;14:429–442. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Sando J. J., Young M. C. Identification of high-affinity phorbol ester receptor in cytosol of EL4 thymoma cells: requirement for calcium, magnesium, and phospholipids. Proc Natl Acad Sci U S A. 1983 May;80(9):2642–2646. doi: 10.1073/pnas.80.9.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Schacht J., Agranoff B. W. Effects of acetylcholine on labeling of phosphatidate and phosphoinositides by ( 32 P) orthophosphate in nerve ending fractions of guinea pig cortex. J Biol Chem. 1972 Feb 10;247(3):771–777. [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Leavitt A. L., Honchar M. P., Hallcher L. M., Phillips B. E. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem. 1981 Jun;36(6):1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Serum stimulation of phospholipase A2 and prostaglandin release in 3T3 cells is associated with platelet-derived growth-promoting activity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):137–141. doi: 10.1073/pnas.77.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. AGEPC (platelet activating factor) induced stimulation of rabbit platelets: effects on phosphatidylinositol, di- and triphosphoinositides and phosphatidic acid metabolism. Biochem Biophys Res Commun. 1982 Jun 15;106(3):697–703. doi: 10.1016/0006-291x(82)91767-3. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I., Martin D. B. Significance of tissue myo-inositol concentrations in metabolic regulation in nerve. Science. 1982 Aug 27;217(4562):848–851. doi: 10.1126/science.6285474. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Myo-inositol transport in the central nervous system. Am J Physiol. 1975 May;228(5):1510–1518. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Schulz I. Regulation of cytosolic free Ca2+ concentration in acinar cells of rat pancreas. Am J Physiol. 1983 Sep;245(3):G347–G357. doi: 10.1152/ajpgi.1983.245.3.G347. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Takai Y., Minakuchi R., Kikkawa U., Sano K., Kaibuchi K., Yu B., Matsubara T., Nishizuka Y. Membrane phospholipid turnover, receptor function and protein phosphorylation. Prog Brain Res. 1982;56:287–301. doi: 10.1016/S0079-6123(08)63780-2. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Masaki T., Goto K. Increase in norepinephrine-induced formation of phosphatidic acid in rat vas deferens after denervation. J Biochem. 1983 Jan;93(1):303–306. doi: 10.1093/oxfordjournals.jbchem.a134169. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Uchida T., Ito H., Baum B. J., Roth G. S., Filburn C. R., Sacktor B. Alpha1-adrenergic stimulation of phosphatidylinositol-phosphatidic acid turnover in rat parotid cells. Mol Pharmacol. 1982 Jan;21(1):128–132. [PubMed] [Google Scholar]
- Varsanyi M., Tölle H. G., Heilmeyer M. G., Jr, Dawson R. M., Irvine R. F. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. EMBO J. 1983;2(9):1543–1548. doi: 10.1002/j.1460-2075.1983.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Receptor-mediated net breakdown of phosphatidylinositol 4,5-bisphosphate in parotid acinar cells. Biochem J. 1982 Sep 15;206(3):555–560. doi: 10.1042/bj2060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Regulation of phosphatidate synthesis by secretagogues in parotid acinar cells. Biochem J. 1982 May 15;204(2):587–592. doi: 10.1042/bj2040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Putney J. W., Jr The relationship of phosphatidylinositol turnover to receptors and calcium-ion channels in rat parotid acinar cells. Biochem J. 1981 Feb 15;194(2):463–468. doi: 10.1042/bj1940463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Rixon R. H., Sikorska M., Tsang B., Walker P. R., Swierenga S. H. The roles of calcium and cyclic AMP in cell proliferation. Ann N Y Acad Sci. 1980;339:216–240. doi: 10.1111/j.1749-6632.1980.tb15980.x. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]


